本笔记内容或有错误,仅供参考
本文 All Right Reserved 保留所有权利,禁止商用和任何形式的转载!
AP 化学的中心就是 原子结构,化合物结构,相互作用力,所有的现象,本质,不同类别,不同主题下的分析都围绕这三个主题展开。
Periodic Table of the Elements 元素周期
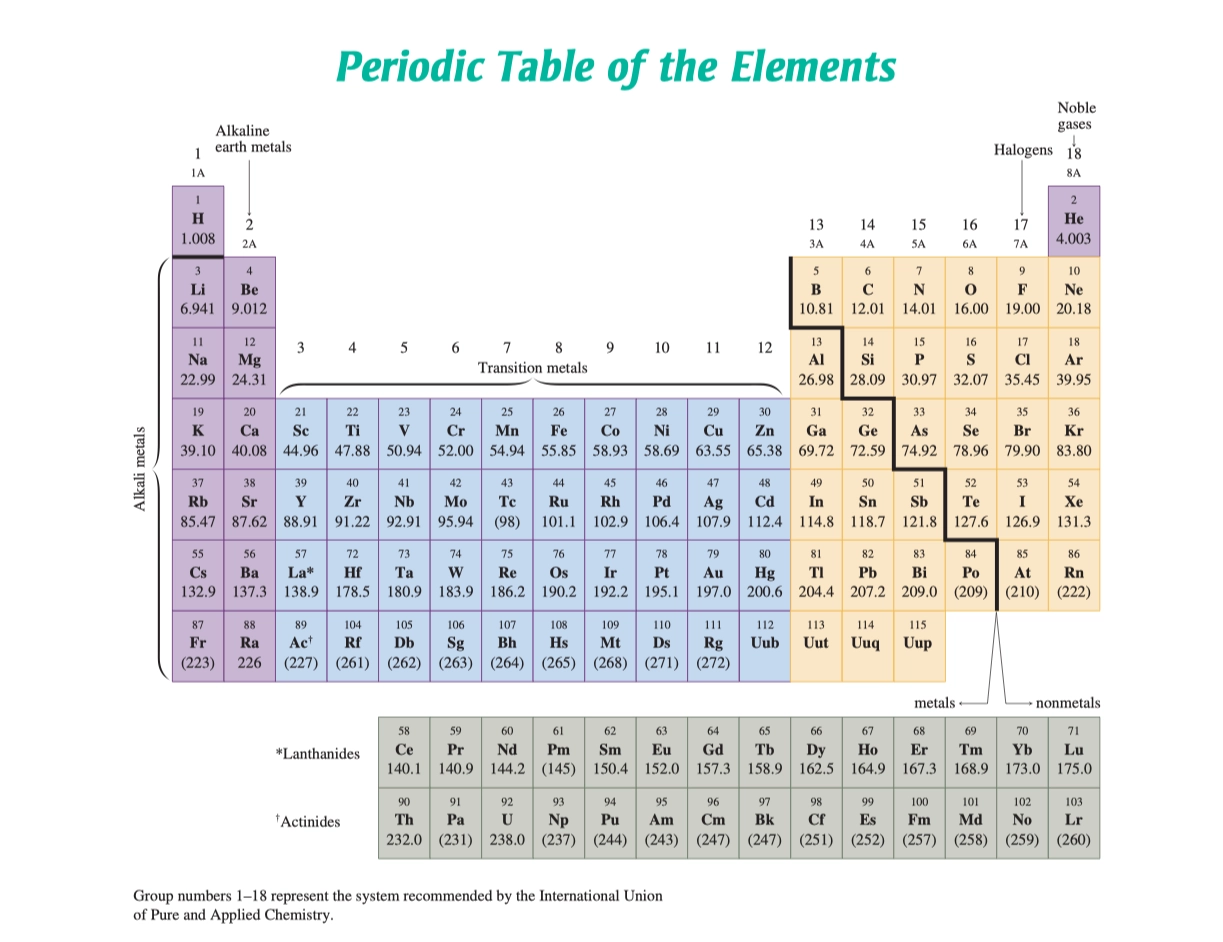
Group 族
- Group IA - 碱金属(Alkali Metals) (\(\ce{Li, Na, K, Rb, Cs, Fr}\)), \(+1\)
- Group IIA - 碱土金属(Alkaline Earth Metals)(\(\ce{Be, Mg, Ca, Sr, Ba, Ra}\)), \(+2\)
- Group VIIA - 卤素(Halogens)(\(\ce{F, Cl, Br, I, At}\))
- Group VIIIA - 稀有气体(Noble Gases)(\(\ce{He, Ne, Ar, Kr, Xe, Rn}\)), \(0\)
- 过渡金属,彩色化合物(Transition Metal, Colored Compound)
- e.g: \(\ce{Fe^{2+}}, \ce{Cu^{2+}}\)
Isotope and Atomic Weight 同位素与原子重量
- \(\text{Atomic Number = Proton Number}\)
- 同位素(Isotope),具有相同质子但不同中子的原子。
- e.g: \(\ce{^{16}O}, \ce{^{18}O}\),
or \(\ce{_{47}^{107}Ag},
\ce{_{47}^{109}Ag}\), or \(\ce{Ag-107},
\ce{Ag-109}\)
- 其中,元素的上标是元素总的质量数
- e.g: \(\ce{^{16}O}, \ce{^{18}O}\),
or \(\ce{_{47}^{107}Ag},
\ce{_{47}^{109}Ag}\), or \(\ce{Ag-107},
\ce{Ag-109}\)
- 通过期望计算相对原子物质:
\[ \text{amu} = \sum\limits_{i=1}^{\mathbb{E}} \mathbb{E}_i \cdot \mathbf{p}_i \] - 其中 \(\mathbb{E}\) 包括所有可能的元素, \(\mathbf{p}\) 代表不同元素的丰度
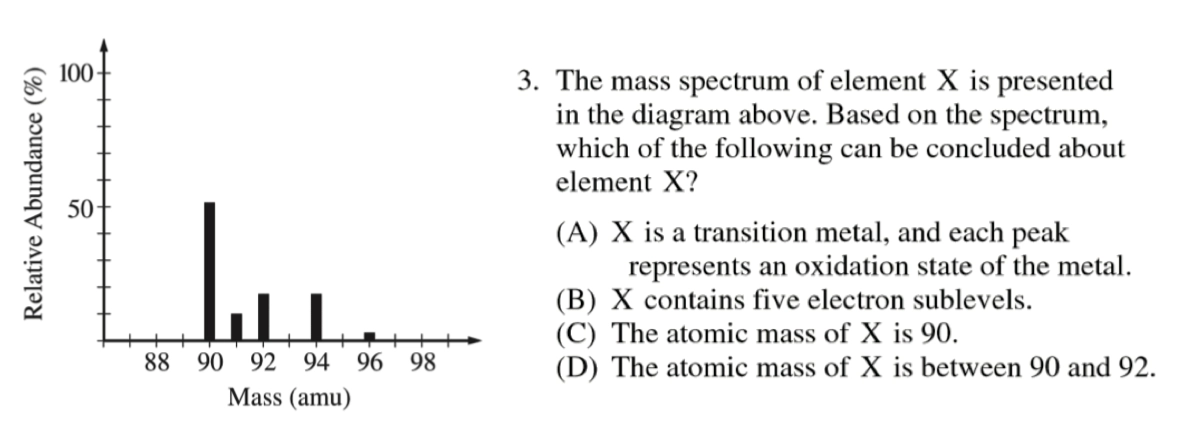
- AP 2015:
D
Diatomic Molecules 双原子分子
- 双原子分子(Diatomic Molecules),包含两个原子组成的分子
- Florine(\(\ce{F_2}\)), Chlorine(\(\ce{Cl_2}\)), Bromine(\(\ce{Br_2}\)), Iodine(\(\ce{I_2}\)), Oxygen(\(\ce{O_2}\)), Nitrogen(\(\ce{N_2}\)), Hydrogen(\(\ce{H_2}\))
Mole 摩尔
- 摩尔(Mole),单位数量的粒子,物质的量
- \(\pu{1 mol} \quad 6.02\times
10^{23}\) particles,
Avogadro's 常数 - \(\pu{1 mol} \; \ce{Fe} \quad 6.02\times 10^{23} \; \ce{Fe}\) atoms
- \(\pu{1 mol} \; \ce{H_2O} \quad 6.02\times 10^{23} \; \ce{H_2O}\) molecules
\[ \text{Moles} = \frac{\text{Grams}}{\text{Molar mass}} = \frac{\text{Particles}}{6.02\times 10^{23}} \]
Formula and Mass Percent 质量比
分子式 \(\ce{C_6H_{12}O_6}\) Glucose (表示出全部的原子数量)
经验公式 \(\ce{CH_2O}\) (最简比)
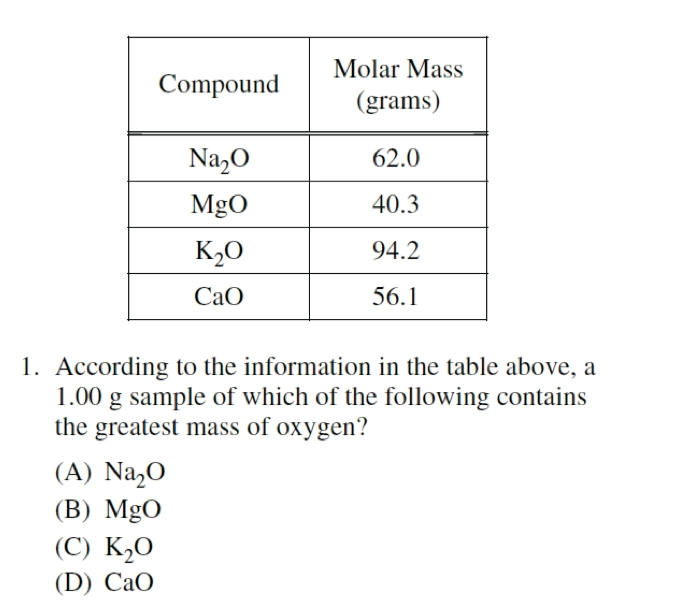
AP 2014:
B
- AP 2013:
A
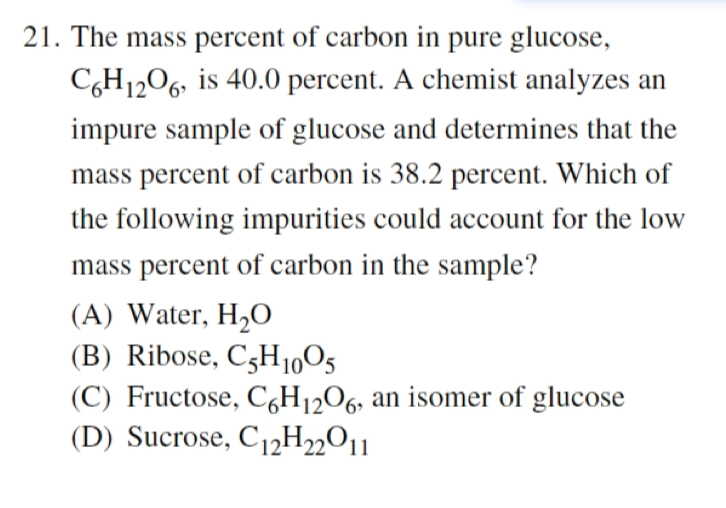
- 首先,\(\ce{C_6H_{12}O_6} = \ce{6(CH_2O)}\)
- A: 不含碳
- B: \(\ce{C_5H_{10}O_5} = \ce{5(CH_2O)}\) 比例成分一致
- C: \(\ce{C_6H_{12}O_6} = \ce{6(CH_2O)}\) 比例成分一致
- D: \(\ce{C_{12}H_{22}O_{11}} \thickapprox \ce{11(CH_2O)}\) 含更多碳 \(\geqslant 40\%\)
Empirical Formula
一个化合物 \(40.00\%\) 碳, \(6.73\%\) 氢, \(53.27\%\) 氧, 确定其经验公式. 其摩尔质量为 \(\pu{60 g/mol}\), 计算其分子式.
假定化合物质量为 \(\pu{100 g}\):
- \(\ce{C}\): \(\frac{\pu{40 g}}{\pu{12 g/mol}} = 3.33\)
- \(\ce{H}\): \(\frac{\pu{6.73 g}}{\pu{1 g/mol}} = 6.73\)
- \(\ce{O}\): \(\frac{\pu{53.27 g}}{\pu{16 g / mol}} = 3.33\)
- \(\ce{C:H:O}\) = \(1 : 2: 1\)
假定经验公式与分子式之间的关系为 \(n\)
- \(n(\ce{CH_2O}) = n\cdot 30 = 2\), so molecular formula is \(\ce{C_2H_4O_2}\)
AP 2018:
B
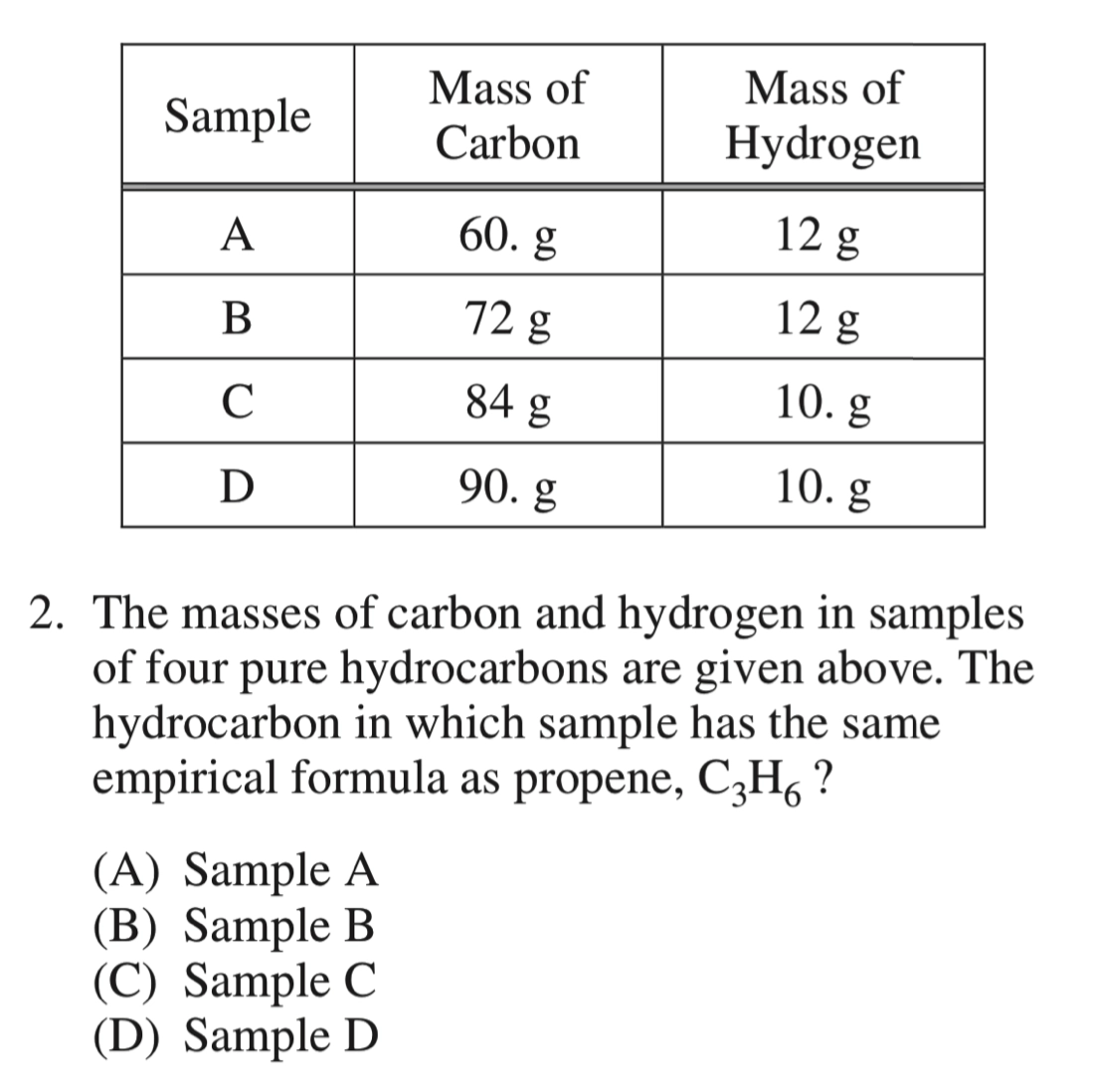
\(\ce{C_3H_6} = \ce{CH_2} \to 12:2 \to 6:1\)
AP Classroom:
D- A \(\pu{42.0 g}\) sample of
compound containing only \(\ce{C}\) and
\(\ce{H}\) was analyzed. The results
showed that the sample contained \(\pu{36.0
g}\) of \(\ce{C}\) and \(\pu{6.0 g}\) of \(\ce{H}\). Which of the following questions
about the compound can be answered using the results of the analysis?
- A: What was the volume of the sample?
- B: What is the molar mass of the compound?
- C: What is the chemical stability of the compound?
- D: What is the empirical formula of the compound?
- 容易错选为
B,实际上我们只能知道最简比,所以摩尔质量可能为最简比形式的倍数,无法确定
- A \(\pu{42.0 g}\) sample of
compound containing only \(\ce{C}\) and
\(\ce{H}\) was analyzed. The results
showed that the sample contained \(\pu{36.0
g}\) of \(\ce{C}\) and \(\pu{6.0 g}\) of \(\ce{H}\). Which of the following questions
about the compound can be answered using the results of the analysis?
AP Classroom:
B- A student obtains a mixture of the chlorides of two unknown metals,
\(\ce{X}\) and \(\ce{Z}\). The percent by mass of \(\ce{X}\) and the percent by mass of \(\ce{Z}\) in the mixture is known. Which of
the following additional information is most helpful in calculating the
mole percent of \(\ce{XCl(s)}\) and of
\(\ce{ZCl(s)}\) in the mixture?
- The number of isotopes of \(\ce{Cl}\)
- The molar masses of \(\ce{X}\) and \(\ce{Z}\)
- The density of either $ $or \(\ce{ZCl(s)}\)
- The percent by mass of \(\ce{Cl}\) in the mixture
- A student obtains a mixture of the chlorides of two unknown metals,
\(\ce{X}\) and \(\ce{Z}\). The percent by mass of \(\ce{X}\) and the percent by mass of \(\ce{Z}\) in the mixture is known. Which of
the following additional information is most helpful in calculating the
mole percent of \(\ce{XCl(s)}\) and of
\(\ce{ZCl(s)}\) in the mixture?
已知 \(\ce{X, Z}\) 的质量分数,求 \(\ce{Cl}\) 的质量分数。
AP 2015 Combustion Analyze 燃烧分析:
B

- \(\pu{12g } \ce{C}\), \(\pu{5g } \ce{H} \to 1:5\)
Coulomb’s Law 库仑定律
- 见 Physics C: EM,我们知道带电粒子之间的作用力为
\[ F = k \frac{q_1q_2}{r^{2}} \]
其中 \(k = \frac{1}{4\pi \epsilon_0}\)
- AP 2015:
C

考虑电荷,排除
A,B考虑原子半径 \(r_\ce{Ca} > r_\ce{Mg}\)
AP 2018:
C
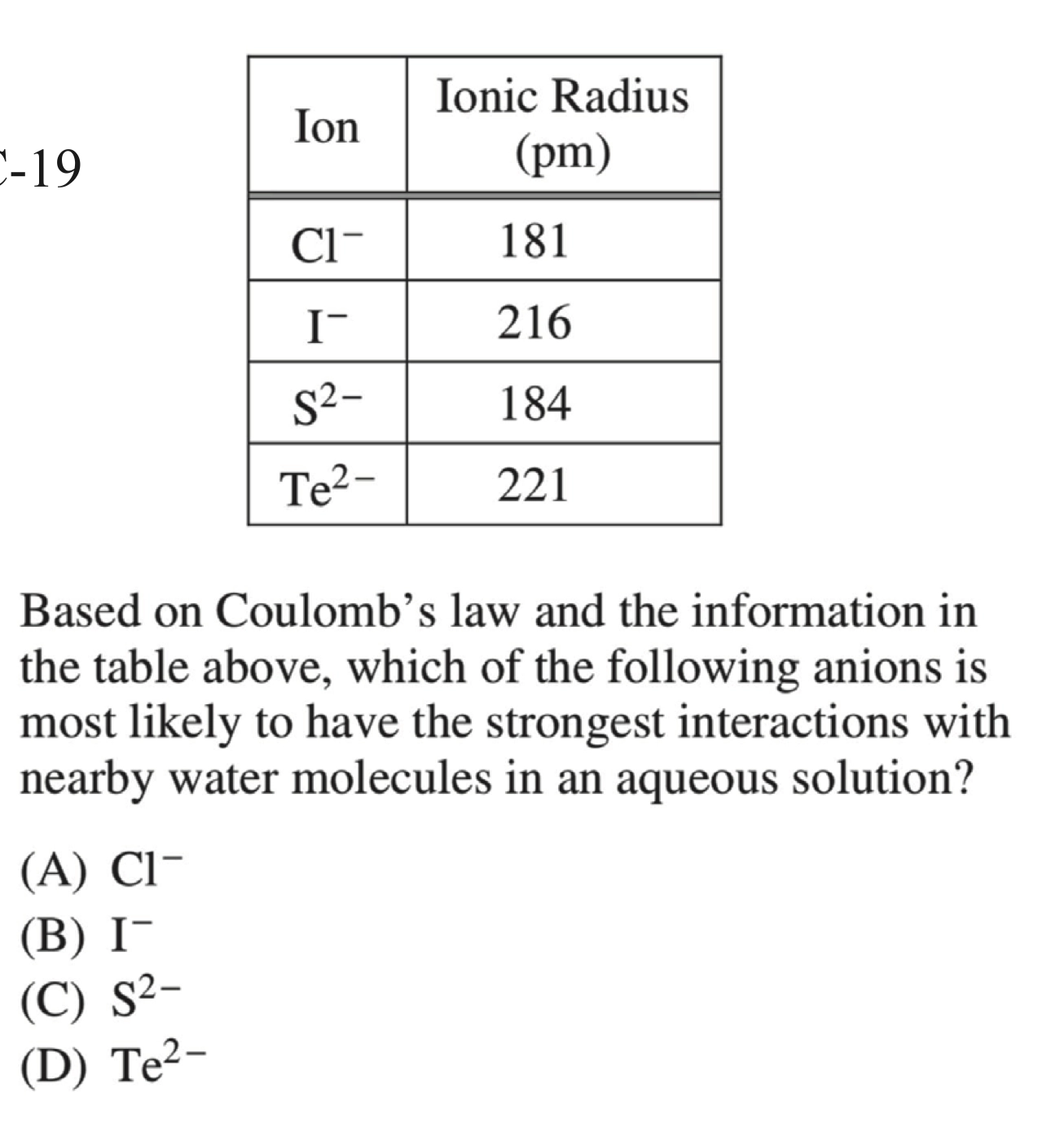
考虑电荷,排除
A,B考虑原子半径 \(r_\ce{Te} > r_\ce{S}\)
AP 2013:
A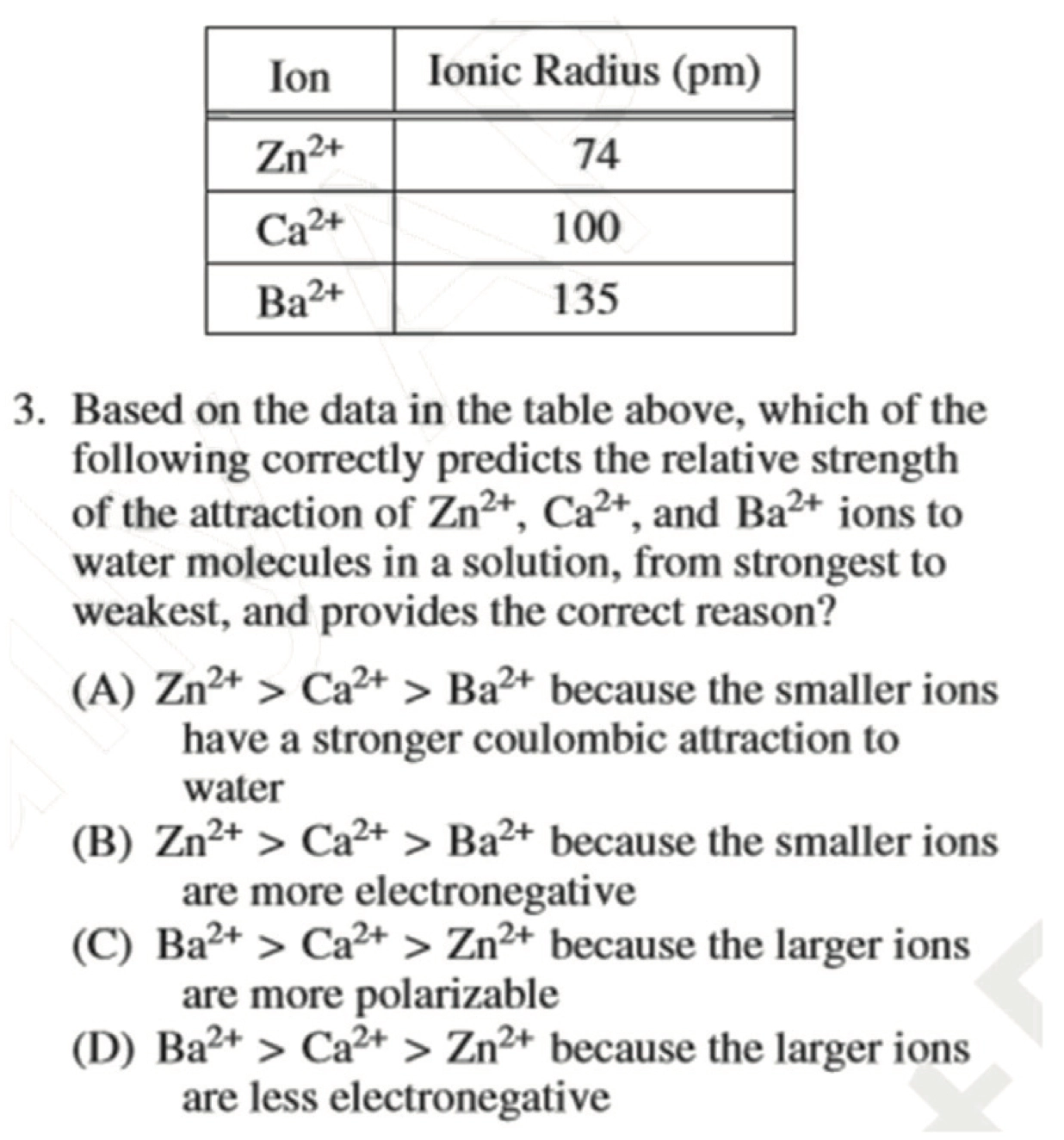
Atomic Structure and Electron Configuration 原子结构与电子轨道排布
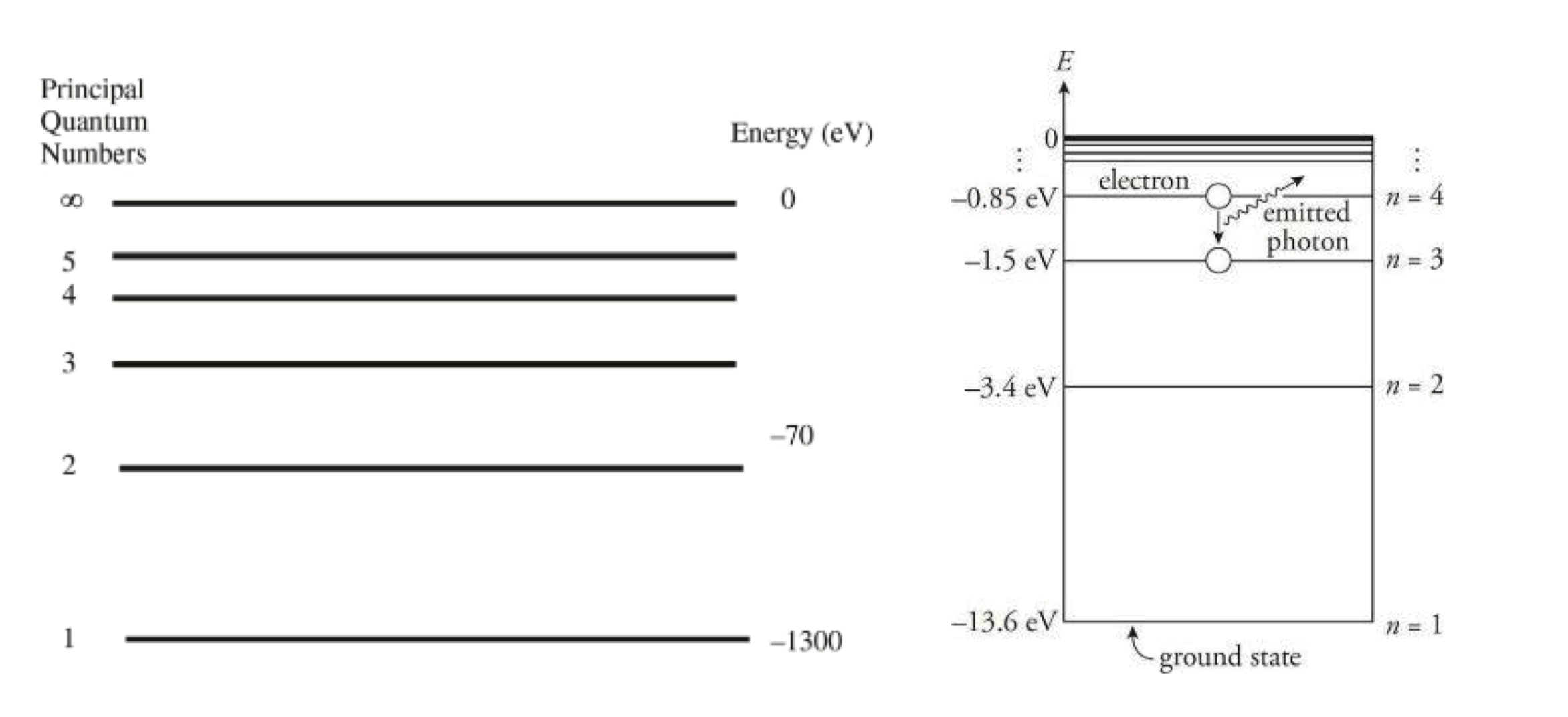
Bohr Model 波尔模型
- 如图所示,当粒子向外能级移动时,粒子吸收能量;当粒子向内移动时,粒子放出能量
- 基态 Ground State: \(\ce{Na}\) 2, 8, 1
- 激发态 Excited State: \(\ce{Na}\)
2, 7, 2
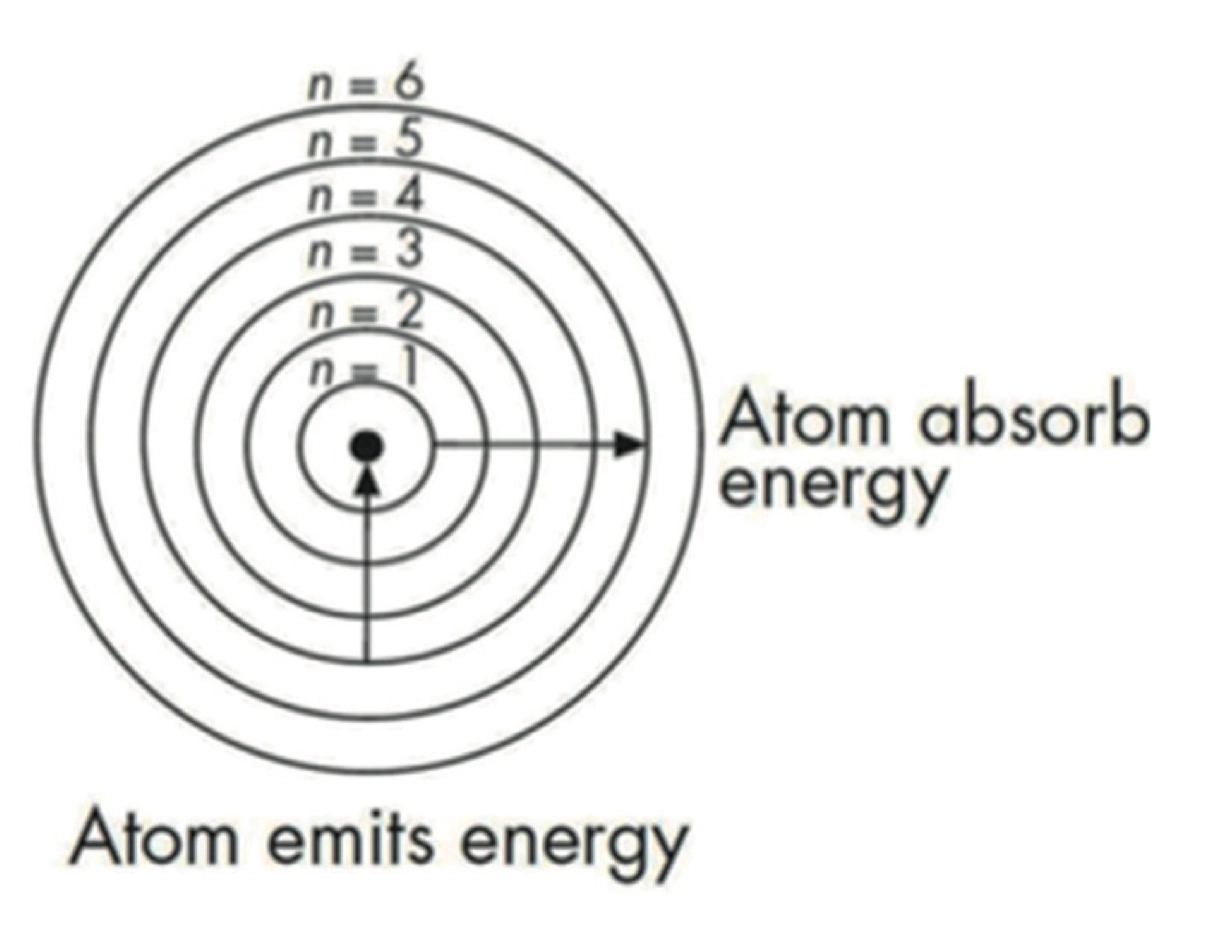
- 电子能级跃迁:
\[ \begin{aligned} \Delta E &= \pu{-2.178e-18J} (\frac{1}{n^{2}_{\text{final}}} - \frac{1}{n^{2}_{\text{initial}}}) \\ \Delta E &= \text{change in energy of the atom (energy of the emitted photon)} \\ n_{\text{final}} &= \text{final level} \\ n_{\text{initial}} &= \text{initial level} \end{aligned} \]
Energy Level 能级
Electromagnetic Radiation 电磁波
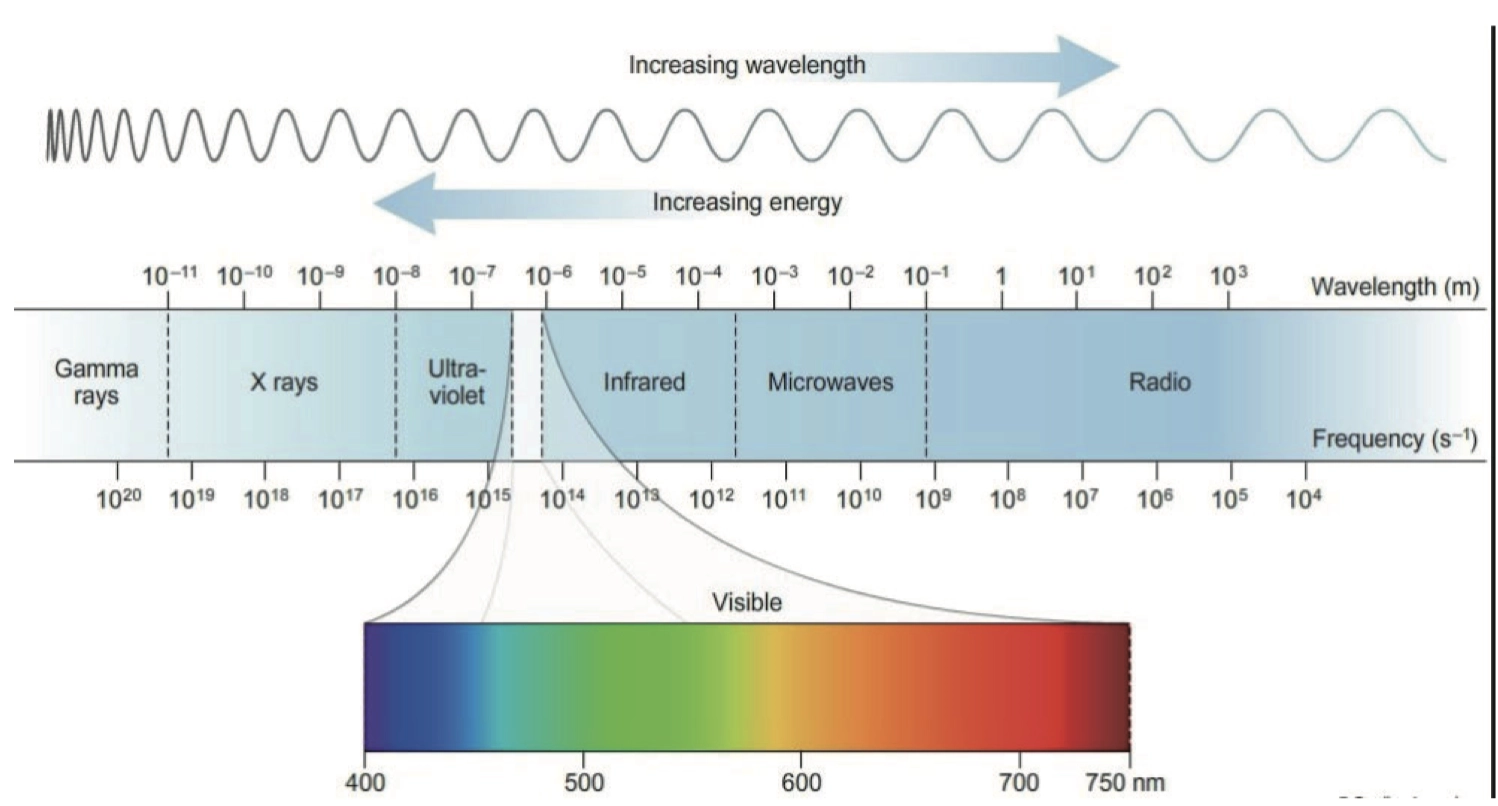
- 频率与波长
\[ c=\lambda v \\ c=\pu{3e8 ms-1} \]
Energy and Electromagnetic Radiation 电磁波与能量
\[ \Delta E = \hbar v = \frac{\hbar c}{\lambda } \]
其中,
\[ \begin{aligned} \Delta E &= \text{energy change of one photon} \\ \hbar &= \text{Planck's constant, } \pu{6.63e-34 J*s} \\ v &= \text{frequency of the radiation} \\ \lambda &= \text{wavelength of the radiation} \\ c &= \text{the speed of light, } \pu{3e8 m / sec} \end{aligned} \]
- Practice:
A
- AP 2015-FRQ-06:
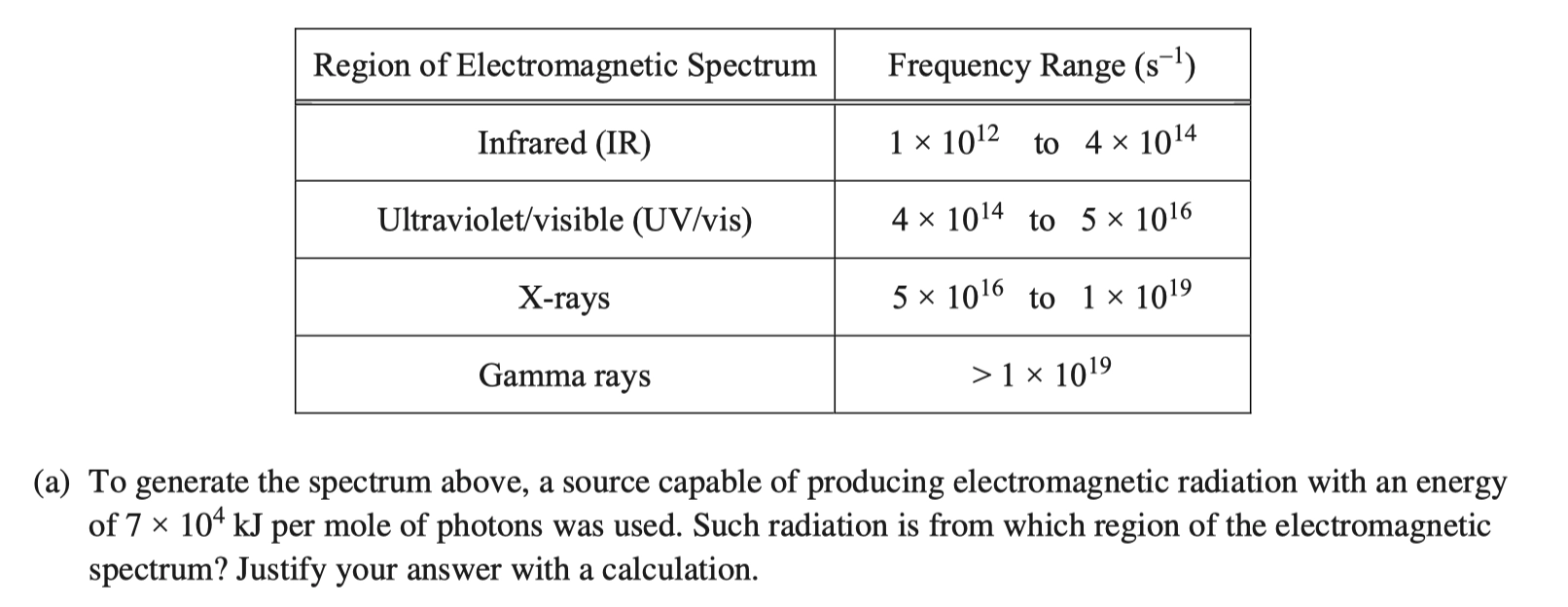
\[ \begin{aligned} \frac{\pu{7e4 kJ}}{\pu{1 mol photons}} \times \frac{\pu{1 mol photons}}{\pu{6.022e23 photons}} &= \pu{1.16e-19 kJ//photon} = \pu{1e-16 J // photon} \\ v = \frac{E}{h} = \frac{\pu{1.16e-16J}}{\pu{6.626e-34 Js}} &= \pu{1.75e17 s-1} = \pu{2e17 s-1} \end{aligned} \] 该电磁波属于 X-ray 范围
Electron Cloud 电子云
- Heisenberg Principle, 海森堡测不准原理
Quantum Theory 量子理论
- Principal Quantum Number 主量子数: \(n\) 壳层 \(1
\leqslant n\)
- 取值如 \(n = 1, 2, 3\)
- Azimuthal Quantum Number 角量子数: \(\ell\) 次壳层 \(0
\leqslant \ell \leqslant n - 1\)
- 若 \(n=3\) 取值如 \(\ell = 0, 1, 2(s, p, d)\)
- Magnetic Quantum Number 磁量子数: \(m_{\ell}\) 能移 \(-\ell \leqslant m_l \leqslant \ell\)
- 若 \(\ell = 2\)
- \(m_{\ell} = -2, -1, 0, 1, 2\)
Shell 壳层
- 随着电子越来越远离原子核,就会存在更多的亚壳层
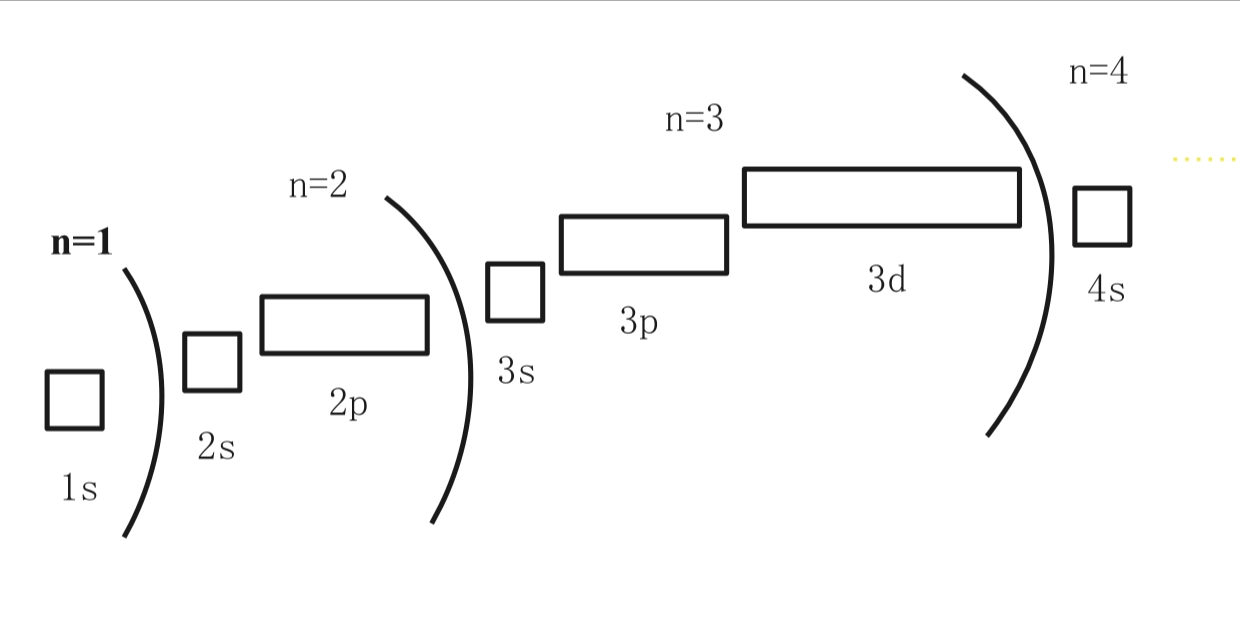
- AP Classroom:
B
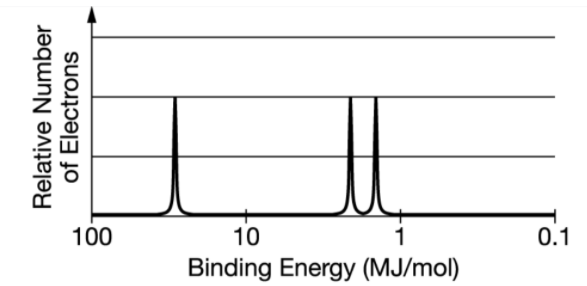
- The complete photoelectron spectrum of the element carbon is
represented above. Which of the following best explains how the spectrum
is consistent with the electron shell model of the atom?
- A: The spectrum shows four electrons in the inner electron shell
- B: The spectrum shows equal numbers of electrons in the three occupied electron subshells.
- C: The spectrum shows that all the electrons in the valence shell have the same binding energy.
- D: The spectrum shows more electrons in the inner electron shell than in the outer electron shell.
- 壳层这个概念容易在记住轨道后忘记
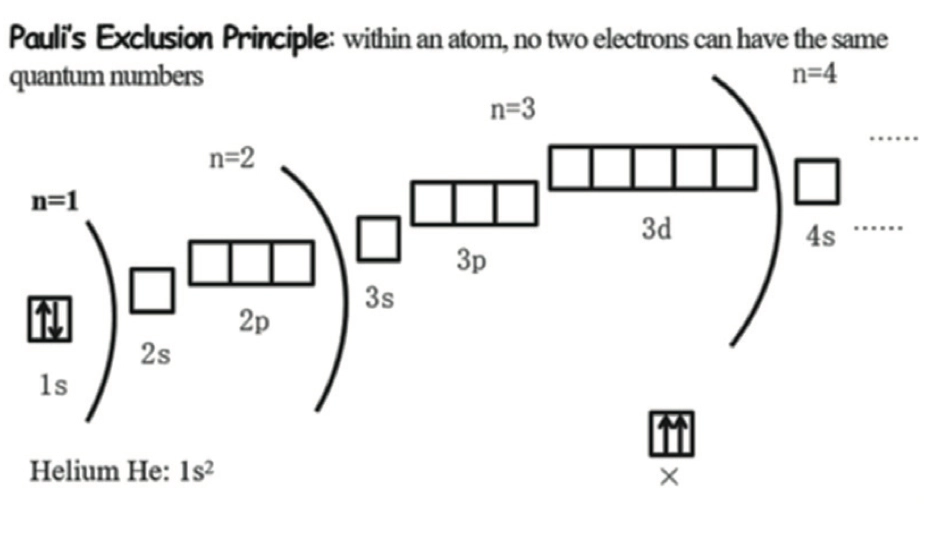
Pauli’s Exclusion Principle 泡利不相容
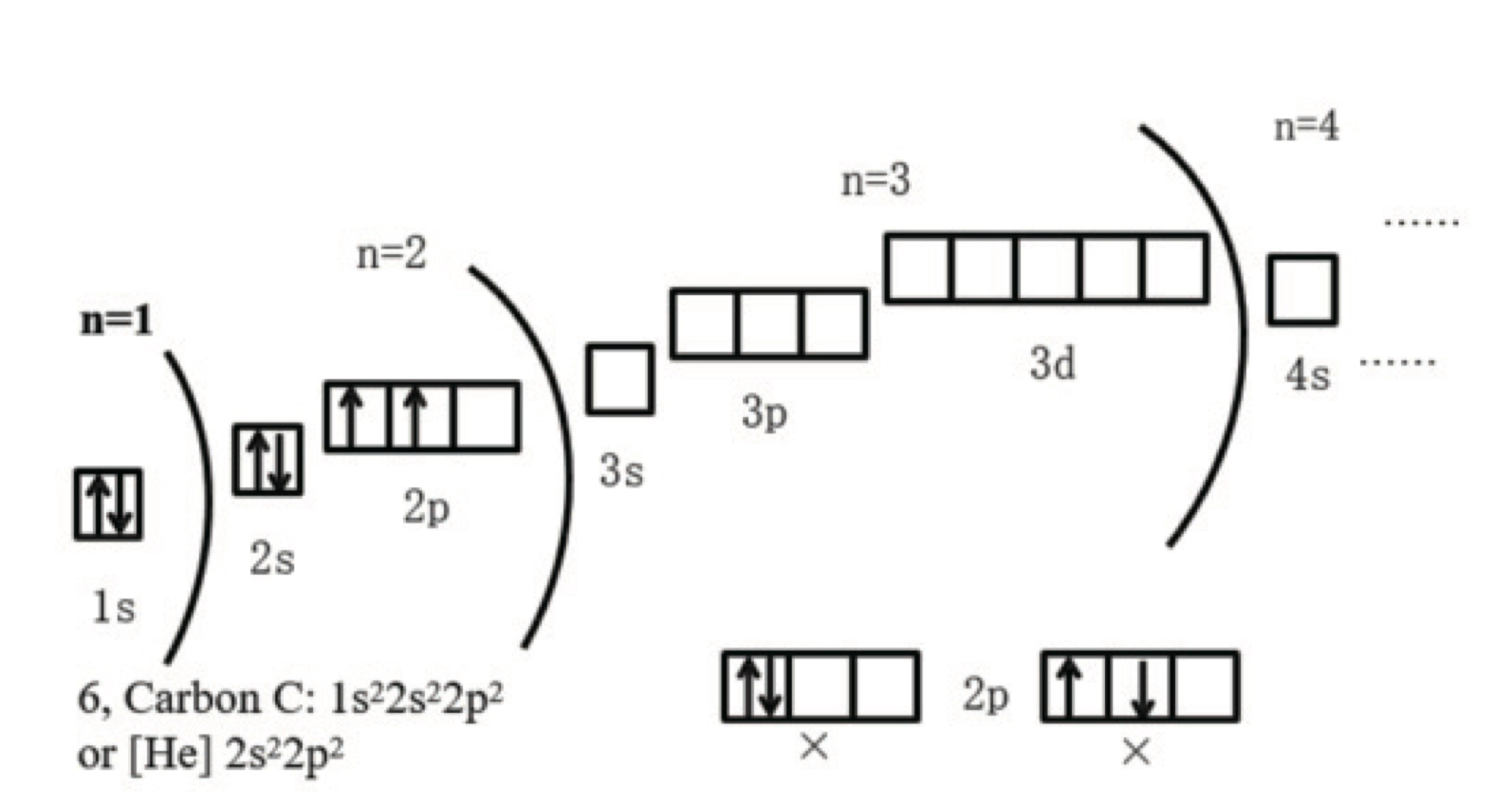
Hund’s Rule 洪特规则
- 电子总会直接占用一整个电子轨道,除非已经没有空轨道了才会去成对
Energy Overlay 能级交错
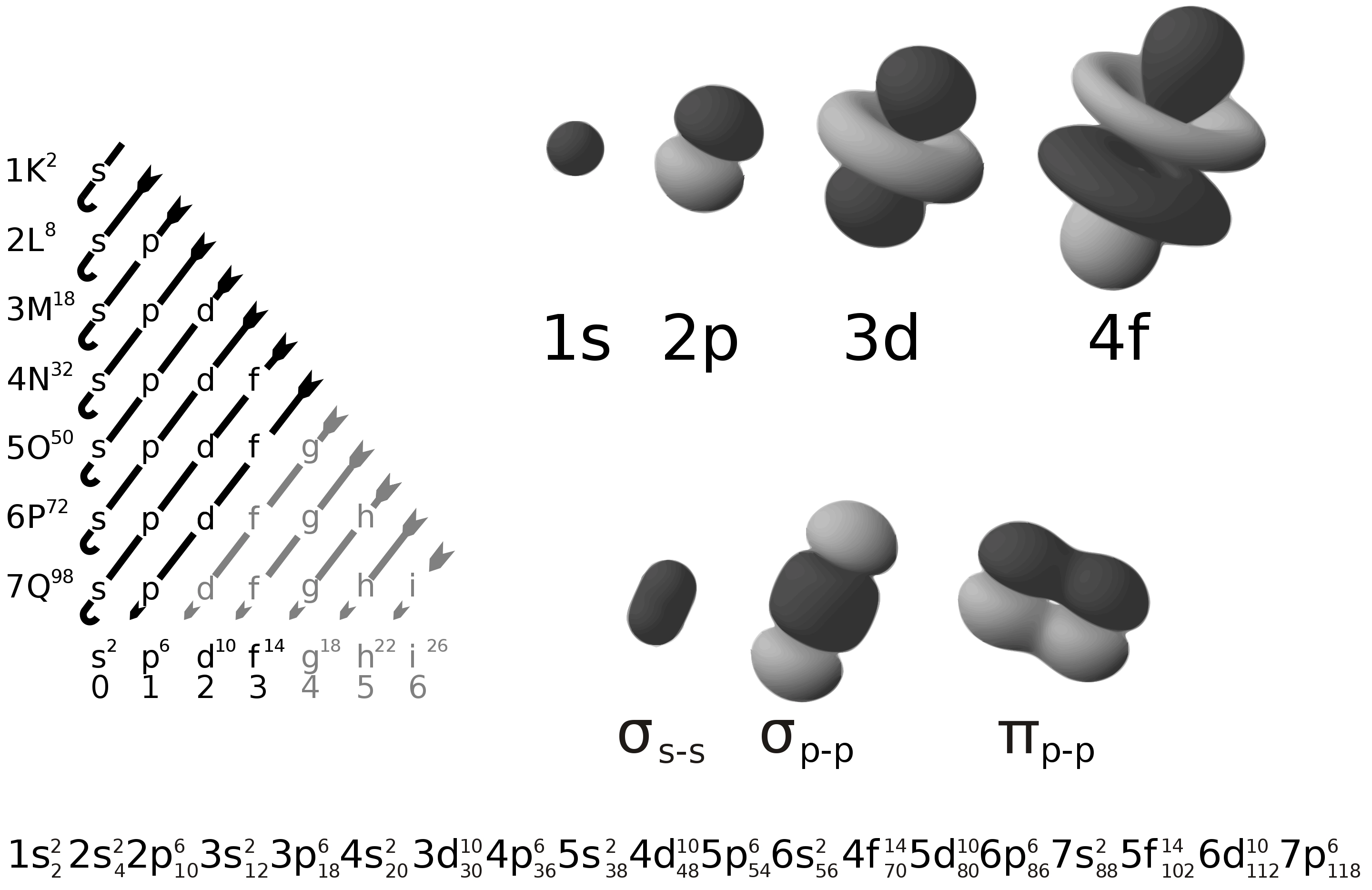
Periodic Block 周期区块
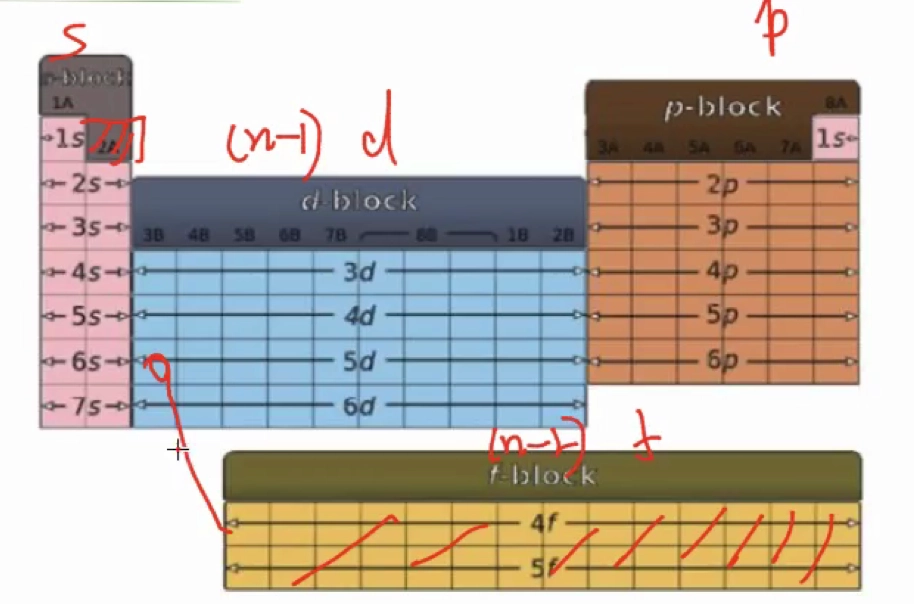
- \(\ce{s, p, (n-1)d, (n-2)f}\)

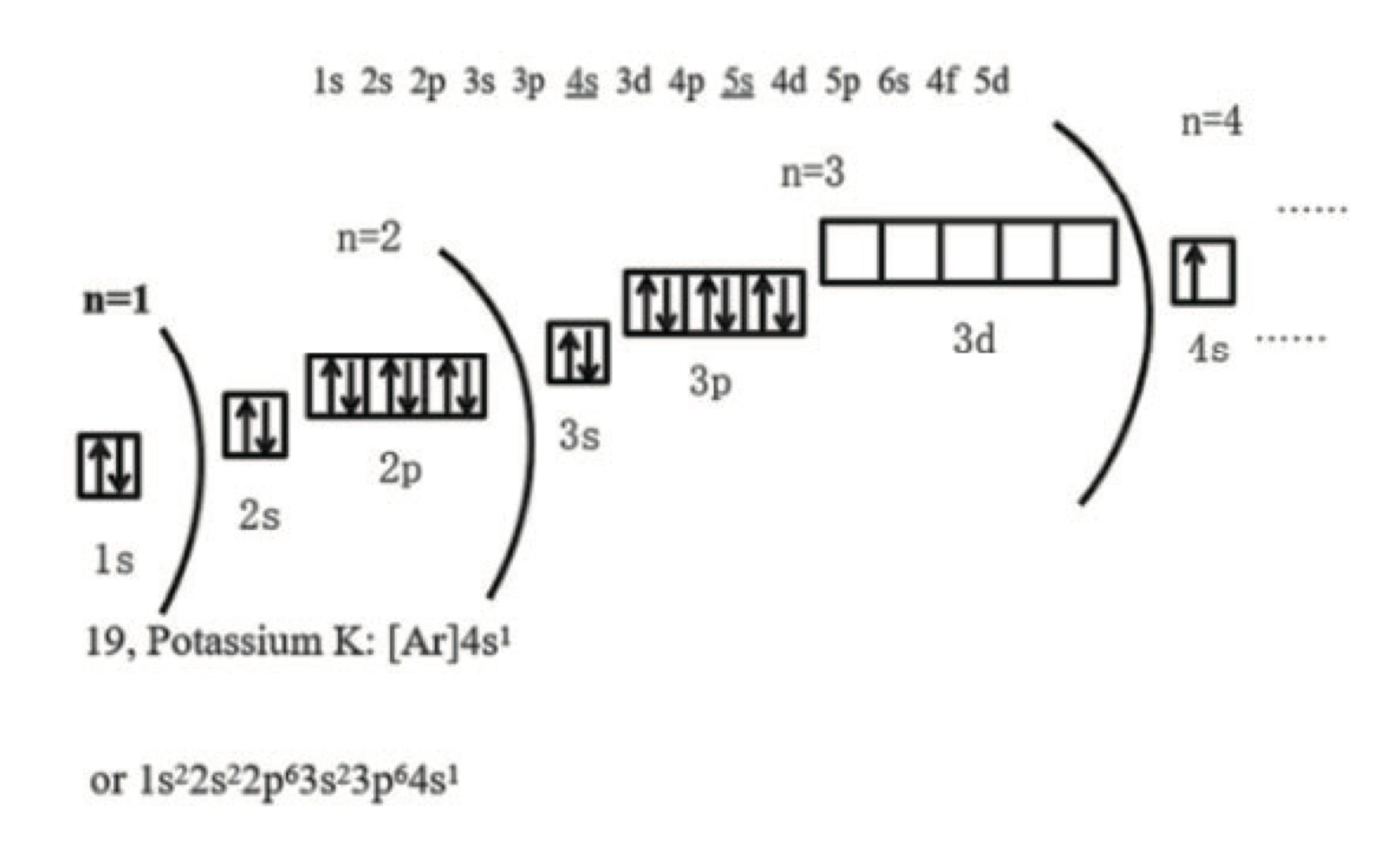
- 通过前一个稀有气体(Nobel Gas)来替代,如 \(\ce{Fe}\) 的核外电子排布可以是 \(\ce{Fe} \quad \ce{1s^{2} 2s^{2} 2p^{6} 3s^{2} 3p^{6} 4s^{2} 3d^{6}} = \ce{[Ar] 4s^{2} 3d^{6}}\)
- 对于镧系元素后而言,如 \(\ce{Pb}\) 的核外电子排布为 \(\ce{Pb} \quad \ce{[Xe] 6s^{2} 4f^{14} 5d^{10} 6p^{2}}\)
Electron Configuration for Ions 离子的电子排布
- 阳离子,先拿走最外面的而不是能量最高的,比如 \(\ce{4s^2}\) 比 \(\ce{3d^6}\) 先走
- 阴离子,直接填充
「最外面」指轨道最外面,参考 Energy Overlay 能级交错
- 所以 \(\ce{Fe^2+}\) 的核外电子排布为 \(\ce{[Ar] 3d^{6}}\),优先拿掉 \(\ce{4s^2}\)
- Practice:
B
Filled & Half-filled 关于全满和半满
- \(\ce{3d^{4} 3d^{9}}\) 没有达到 半满(Half-filled),或是 全满(Filled) 的稳定结构,从 \(\ce{s}\) 轨道上抢电子,去使得结构更稳定。
- 观察以下两个元素的电子轨道排布,电子倾向于形成稳定的半满与全满的 \(\ce{d}\) 轨道
- \(\ce{Cr} \quad = \ce{[Ar] 4s^{1} 3d^{5}} \neq \ce{[Ar] 4s^{2} 3d^{4}}\)
- \(\ce{Cu} \quad = \ce{[Ar] 4s^{1} 3d^{10}} \neq \ce{[Ar] 4s^{2} 3d^{9}}\)
- Practice:
A
Photoelectron spectroscopy 光电子能谱
和 Bohr Model 中的 Energy Level 很相似
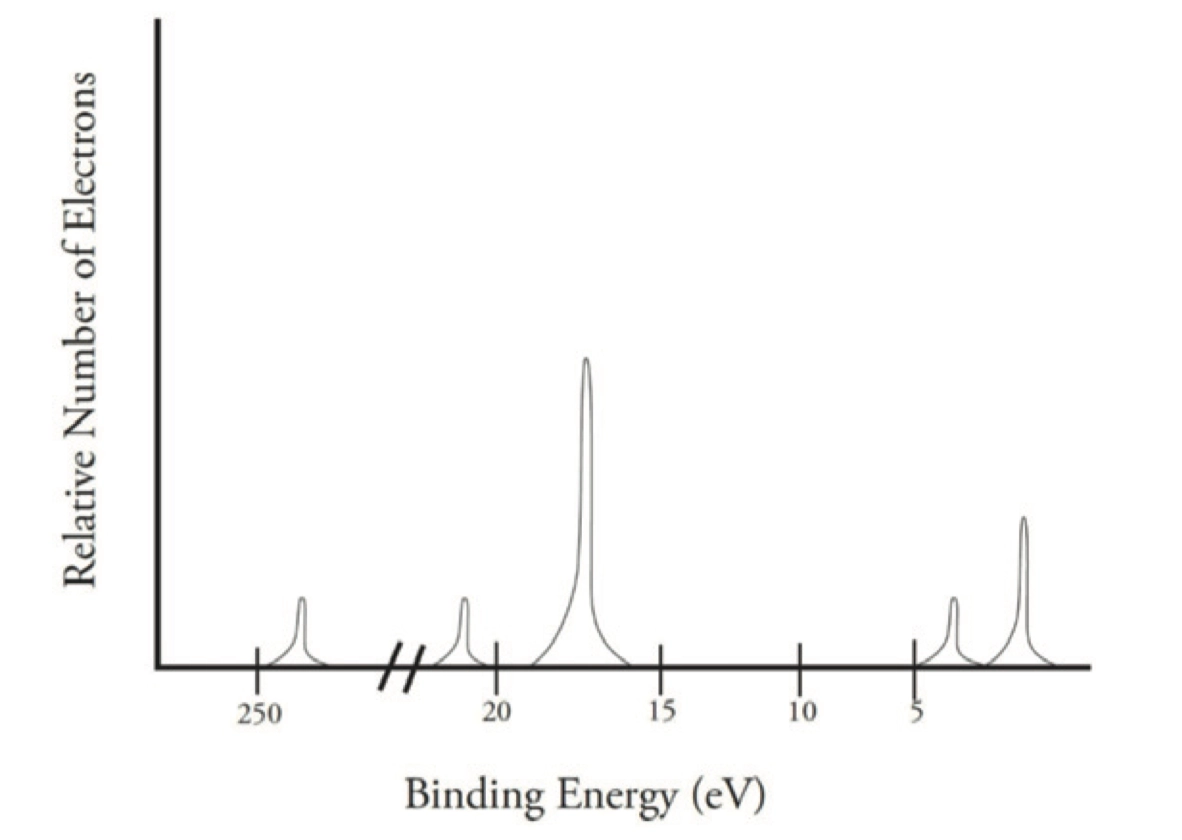
横坐标结合能(Binding Energy),越高离原子核越近,越低离原子核越远,表示激发一个电子所需的能量
纵坐标的激发电子数,越高激发的电子越多,也就是特定轨道上的电子数目更多
每一个波峰对应一个亚层(Sublevel),用于分析元素种类
Practice:
B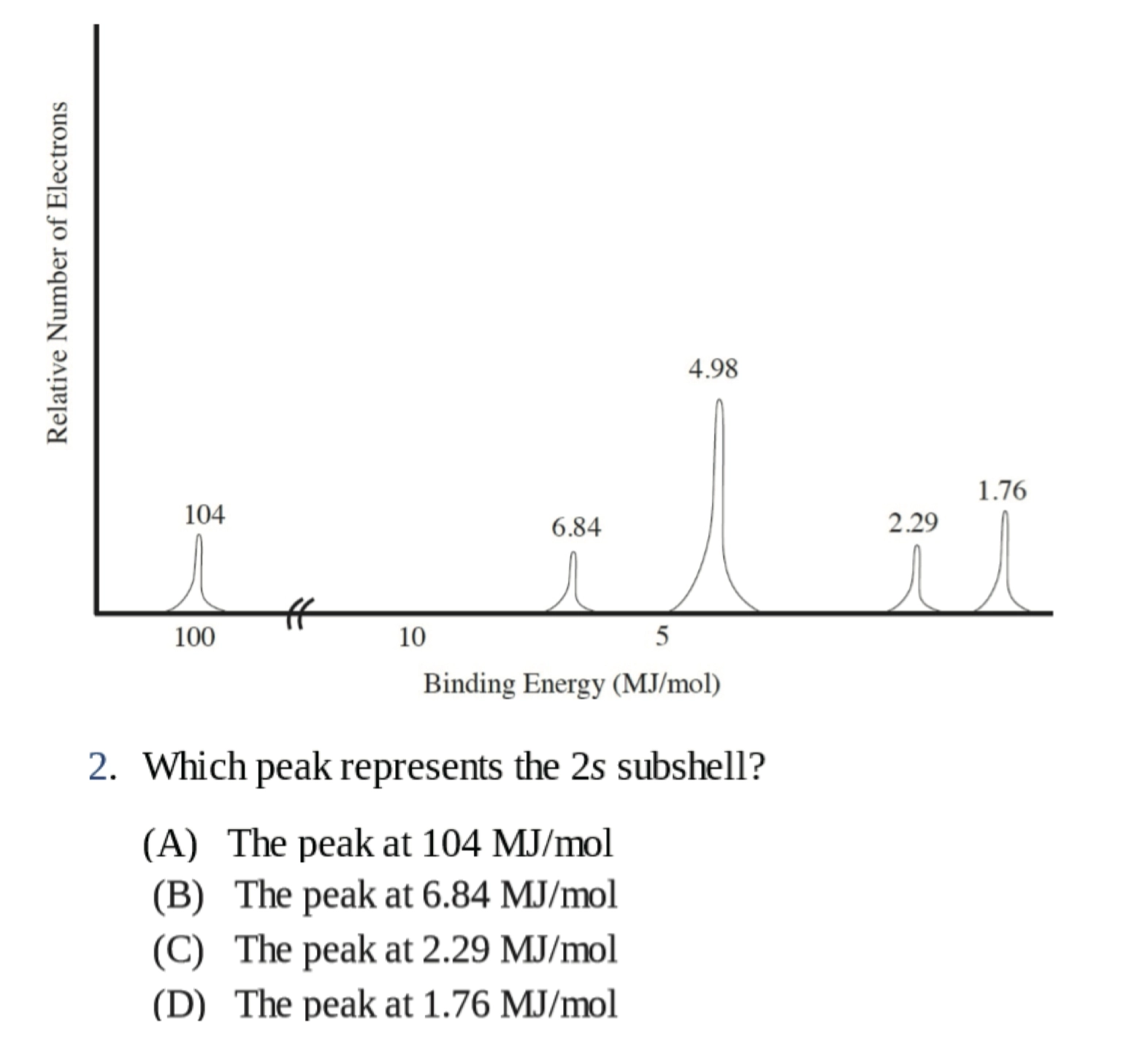
Practice:
D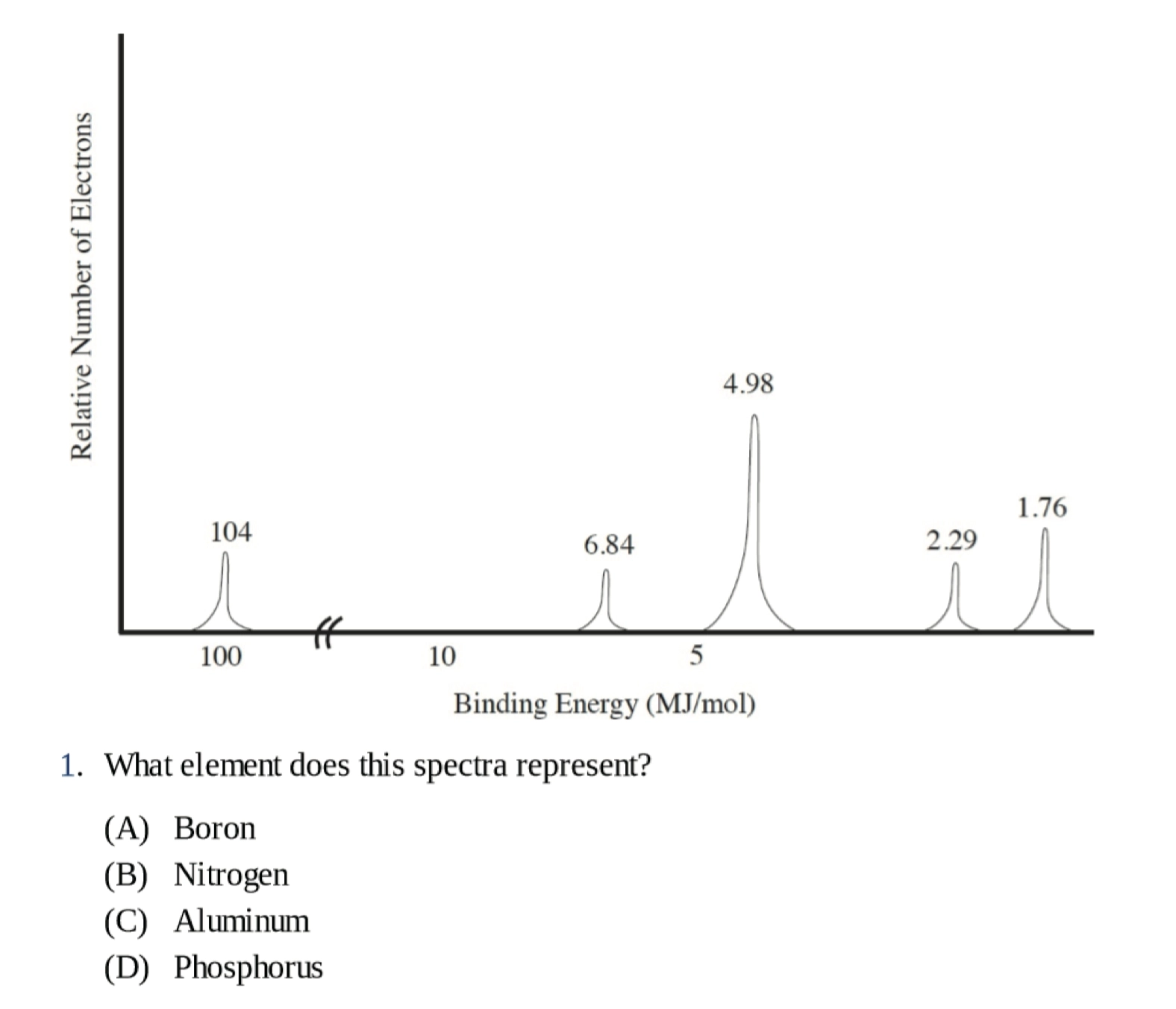
\(\ce{1s^{2} 2s^{2} 2p^{6} 3s^{2} 3p^{>2}}\)
元素原子核中的质子所带正电荷会影响轨道所需要的结合能(吸引负电荷电子 \(\ce{e-}\)),如 \(\ce{N}\) 和 \(\ce{O}\) 在 \(\ce{1s}\) 轨道所需要的结合能便是 \(E_{\ce{O}} > E_{\ce{N}}\)
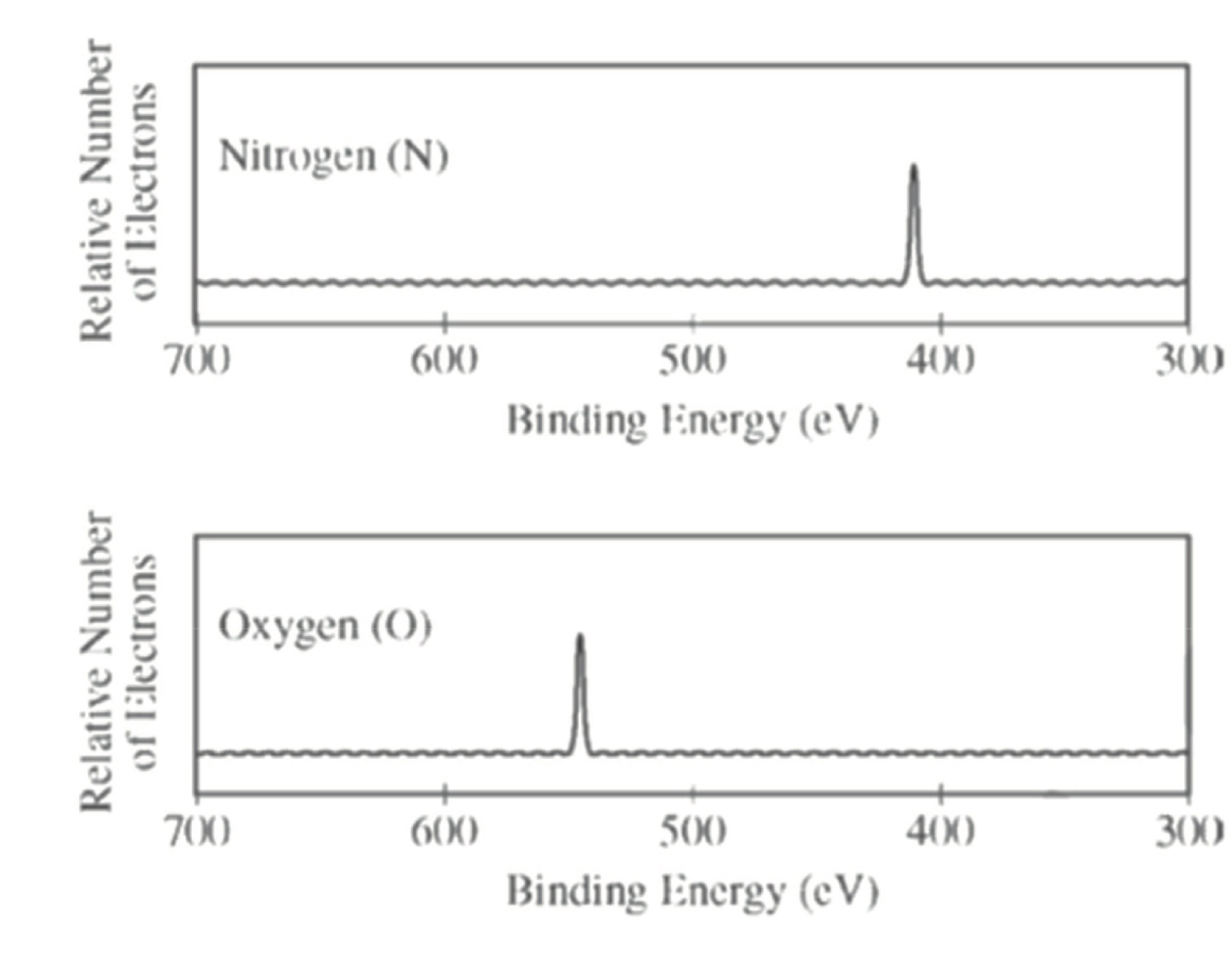
Practice:
D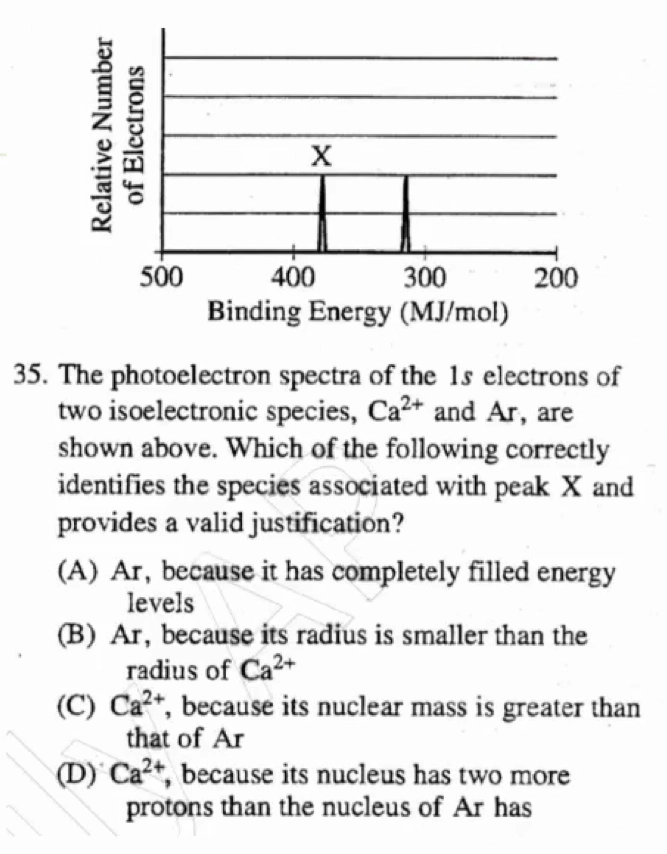
- \(\ce{Ca^2+}\) 比 \(\ce{Ar}\) 含有更多的正电荷质子, 所以 \(\ce{Ca^2+}\) 在 \(1s\) 轨道上有更强的吸引力.
光电子能谱所发射的能量符合如下规则
\[\text{Incoming Radiation Energy} = \text{Binding Energy} + \text{Kinetic Energy(of the ejected electron)}\]
Practice:
D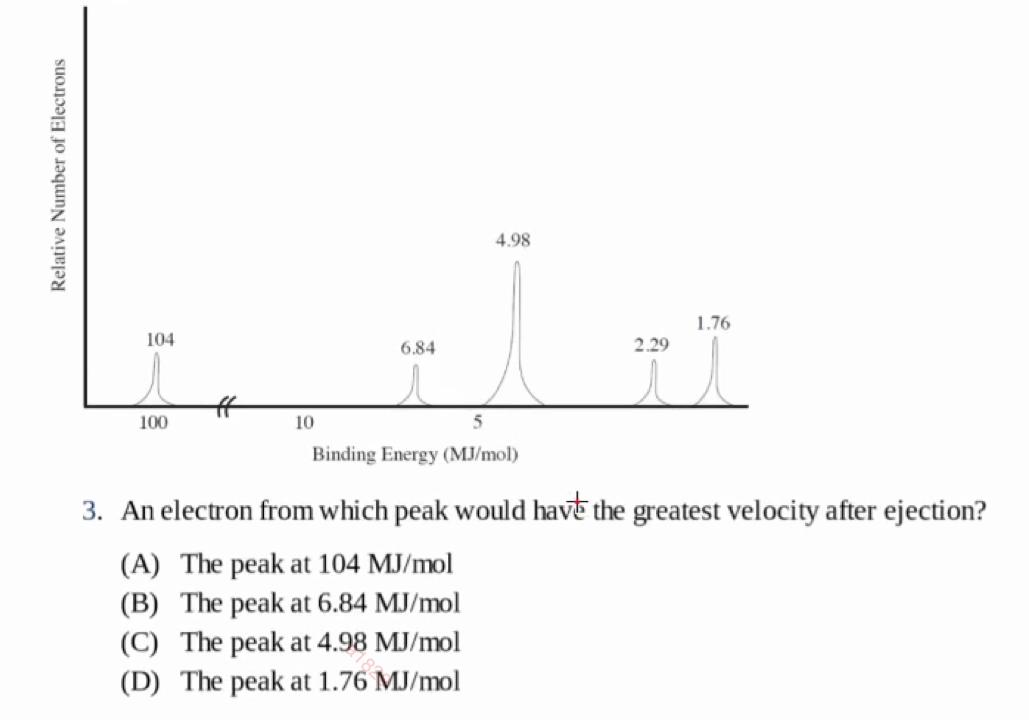
- D,只需要克服最小的结合能,剩下所有能量转化为动能发射出去
Practice:
C
\(\text{Incoming Radiation Energy} = \text{Binding Energy} + \text{Kinetic Energy(of the ejected electron)} = \pu{2.5 V} + \pu{2 V} = \pu{4.5 V}\)
Practice :
A
\(c = \lambda v\), 越短的波长 \(\lambda\) 意味着更高的频率 \(v\), 使得能量更大 \(E = \hbar v\)
Practice:
D
光强只能使得发射的光子变多,所以光强不会影响光发射的每份能量 \(E = \hbar v\)
Absorption Spectrum 吸收光谱
紫外线,可见光(Ultraviolet / Visible Radiation) 与电子的能级跃迁(Transitions in Electronic Energy Level)有关 (用于探测电子排布结构)
红外线(Infrared Radiation) 与 分子震动(Molecular Vibrations)有关(用于探测分子不同类型的键)
微波(Microwave Radiation) 与 分子自旋有关(Molecular Rotations) (用于研究分子形状 Molecular Shape)
AP 2015:
D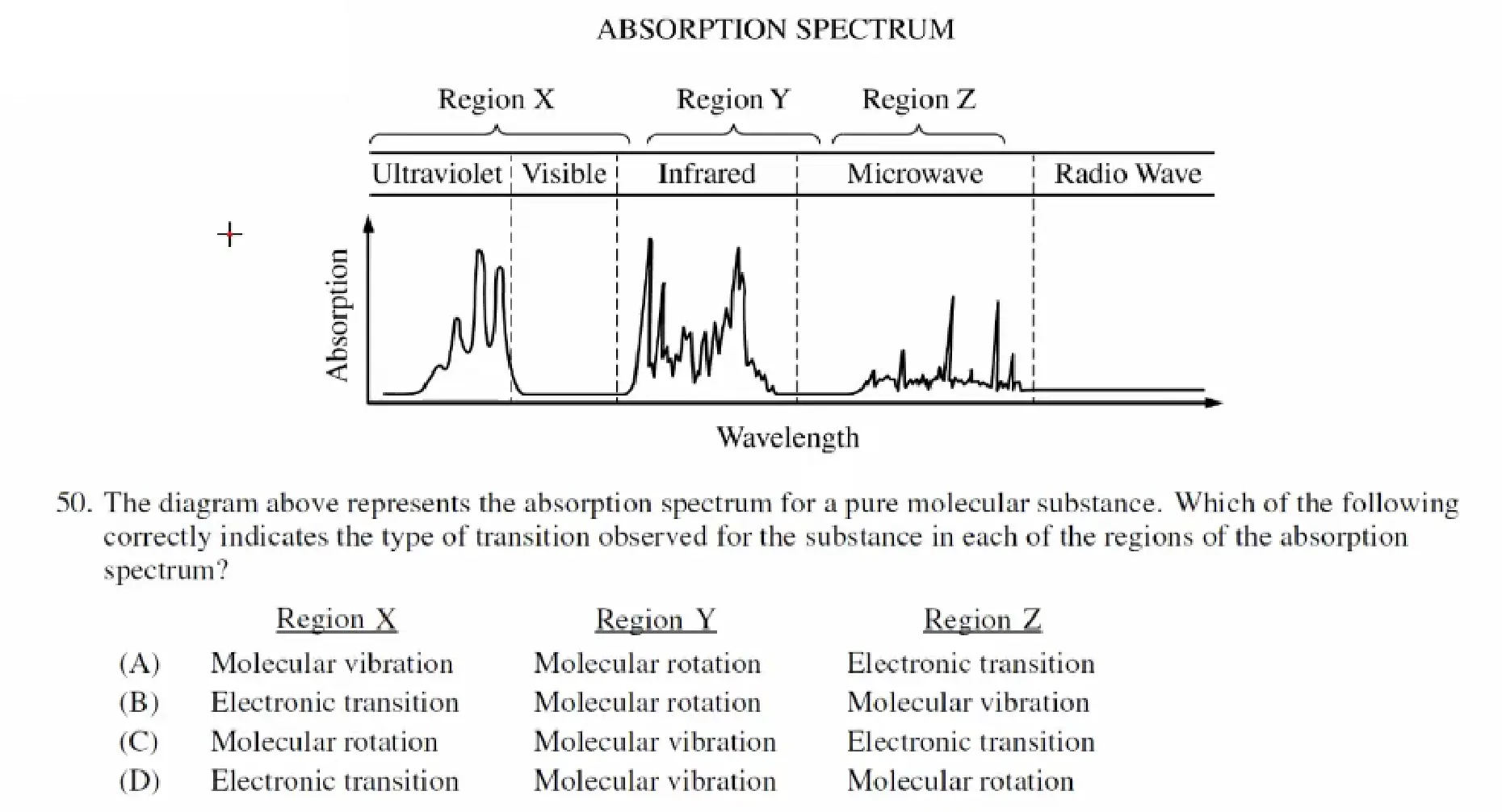
Practice:
C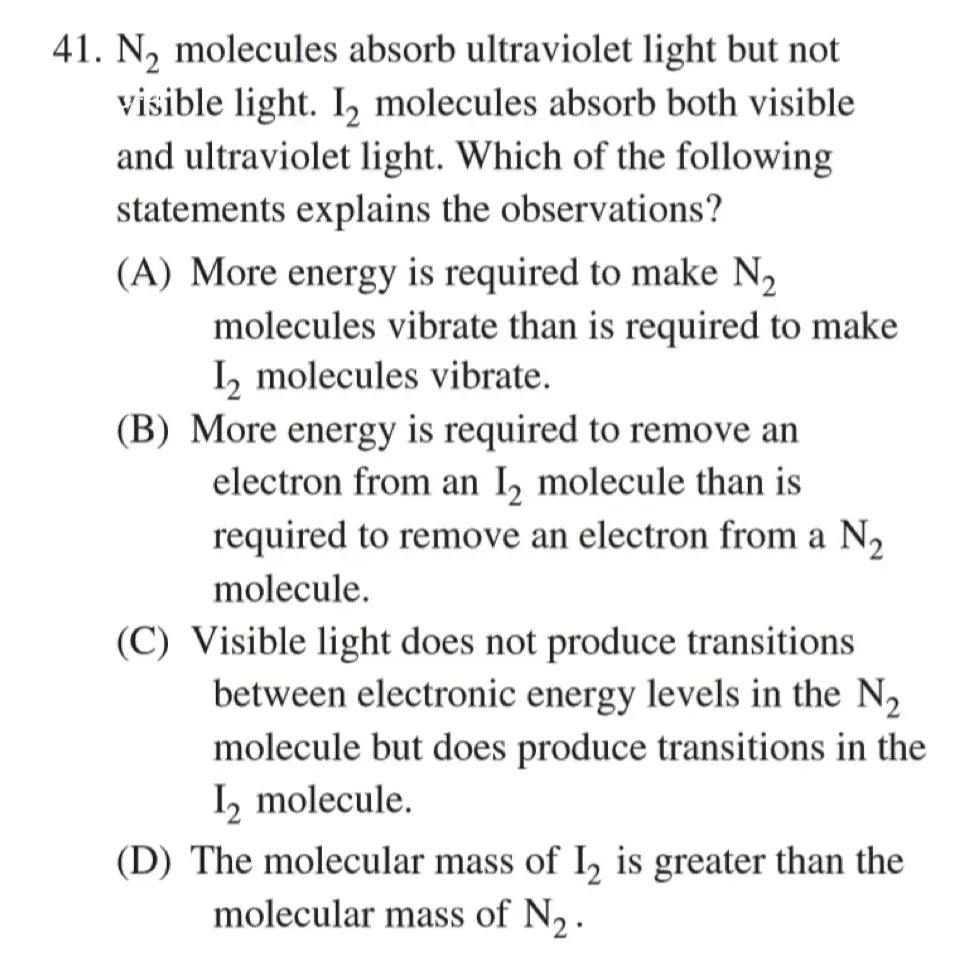
紫外线能量高,可见光能量低
对于 \(\ce{N2}\) 来说,只有两个层级,能量差大,跃迁需要的能量高
对于 \(\ce{I2}\) 来说,有多个能级,外层能级的能量差小,跃迁需要的能量低
Periodic Trend 周期趋势
Atomic Radius 原子半径
总体趋势,朝左下增加
从左到右: 下降
- 质子数更多,对整体吸引力越大
由上到下: 增加
- 占用了更多电子壳层,半径变大
对于同一个元素: 由电子数量决定
- 电子越多,越大的电子间斥力
- More number of electrons, greater electron-electron repulsion
- 电子越多,越大的电子间斥力
对于相同的电子轨道排布: 由质子数决定
- 质子越多,对电子越大的吸引力,越小的半径
- More number of protons, greater attraction between the nuclear charges and electrons and smaller radius.
- 质子越多,对电子越大的吸引力,越小的半径
Practice:
D

- AP Classroom:
C

- The atomic radii of the elements in the nitrogen group in the
periodic table are given in the table above. Which of the following best
helps explain the trend of increasing atomic radius from \(\ce{B}\) to \(\ce{Bi}\)?
- A: The number of particles in the nucleus of the atom increases.
- B: The number of electrons in the outermost shell of the atom increases
- C: The attractive force between the valence electrons and the nuclei of the atoms decreases.
- D: The repulsive force between the valence electrons and the electrons in the inner shellis decreases.
Ionization Energy 电离能
- 电离能(Ionization Energy)定义为电离能是从气态原子中取出电子所需要的能量。
- 电子从基态移到 \(n = \infty\) 所需要的能量
\[ \ce{Na(g)} \to \ce{Na+(g)} + \ce{e-} \]
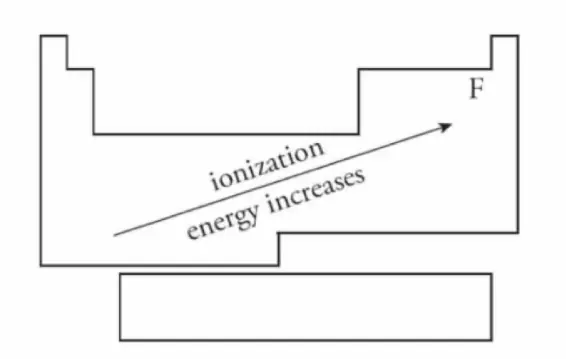
- 与原子半径完全相反
- 从左到右: 增加
- 质子数更多,对整体吸引力越大,所以移走一个电子更难
- 由上到下: 下降
- 占用了更多电子壳层,半径变大,所以移走一个电子更简单
- 可能会认为,从上到下,质子数不是更多了吗?吸引力为什么不会更大? ### Shielding Effect 屏蔽效应
- 电子在原子内相互之间会排斥,所以壳层越多虽然核电荷数更大了,但是屏蔽效应会使得内壳层与外壳层之间相互排斥,所以从上到下电离能减少。
Effective Nuclear Charge 等效核电荷
- 等效核电荷数,指 电子在屏蔽效应下真正受到的核电荷的吸引数有多少, e.g \(\ce{O}\): \(8 - 2 = 6\)
\[ \begin{aligned} Z_{\text{eff}} &= Z - S \\ Z &= \text{atomic number} \\ S &= \text{number of shielding(inner) electrons} \end{aligned} \]
- AP 2014:
D

C虽然没什么大问题,但不是本质的解释,反过来也可以说 \(\ce{Cl}\)2-7排布,不愿意马上就要稳定了,更需要得电子,也不愿意失去AP Classroom:
D

- 等效核电荷也能用来解释原子半径,从左向右等效核电荷是增加的,从上到下的等效核电荷数是不变的。
Big Jump of Ionization Energy 电离能的剧变
当电离能出现了剧烈增加,表示发生了跨层
比如第二电离能到第三电离能的剧烈增加,那么最外层电子就是两个
Practice:
B
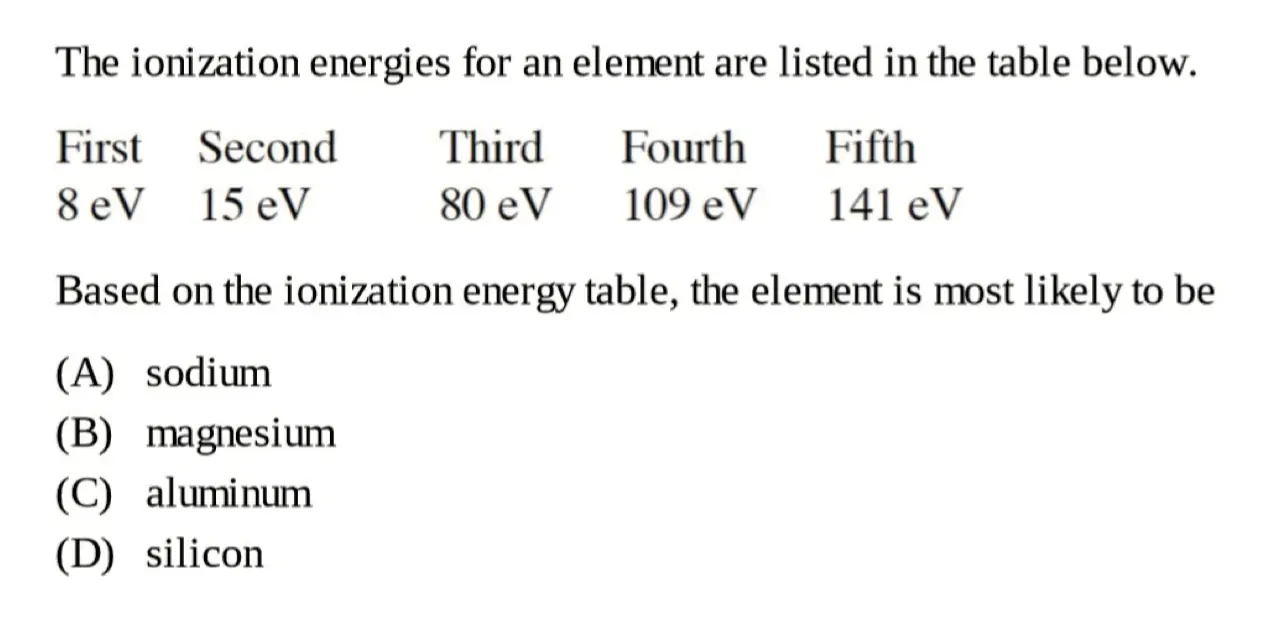
第二到第三的剧烈变化(说明最外层电子数为 \(2\)),考虑第二主族元素,选
BAP 2018:
C
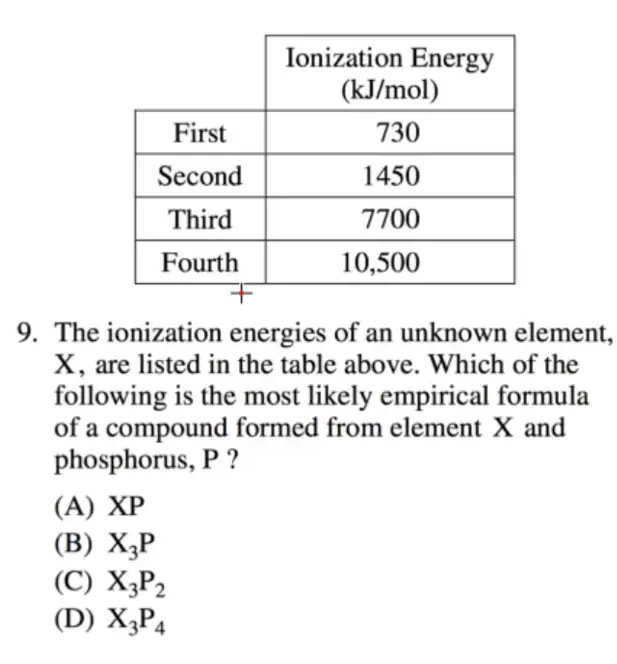
- 第二到第三的剧烈变化,考虑价电子为 \(2\),选
C
Exception of Periodic Trend 周期特例
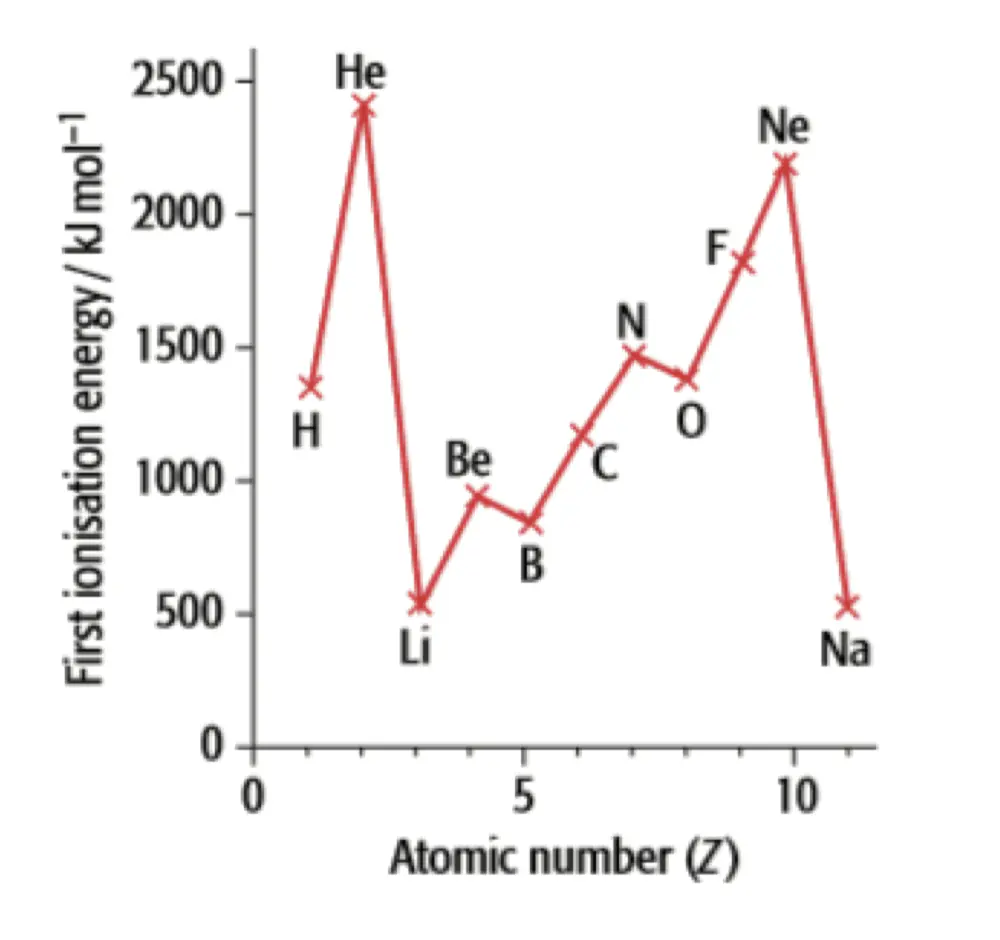
- 观察上图,发现 \(\ce{Be}, \ce{B}\),\(\ce{N}, \ce{O}\) 与周期性变化规律不一样
- 考虑 \(\ce{N}\) 的 电子轨道排布
\(\ce{1s^{2} 2s^{2} 2p^{3}}\)
- \(\ce{O}\) 的 电子轨道排布 \(\ce{1s^{2} 2s^{2} 2p^{4}}\)
- 发现,$ $ 开始出现成对电子,成对电子相互之间会排斥(如同 Shielding Effect)
- 考虑 \(\ce{Be}\) 的电子轨道排布
\(\ce{1s^{2} 2s^{2}}\) 全满,稳定
- \(\ce{B}\) 的电子轨道排布 \(\ce{1s^{2} 2s^{2} 2p^{1}}\) 不稳定,更愿意失电子,只要更少的电离能就能移走电子
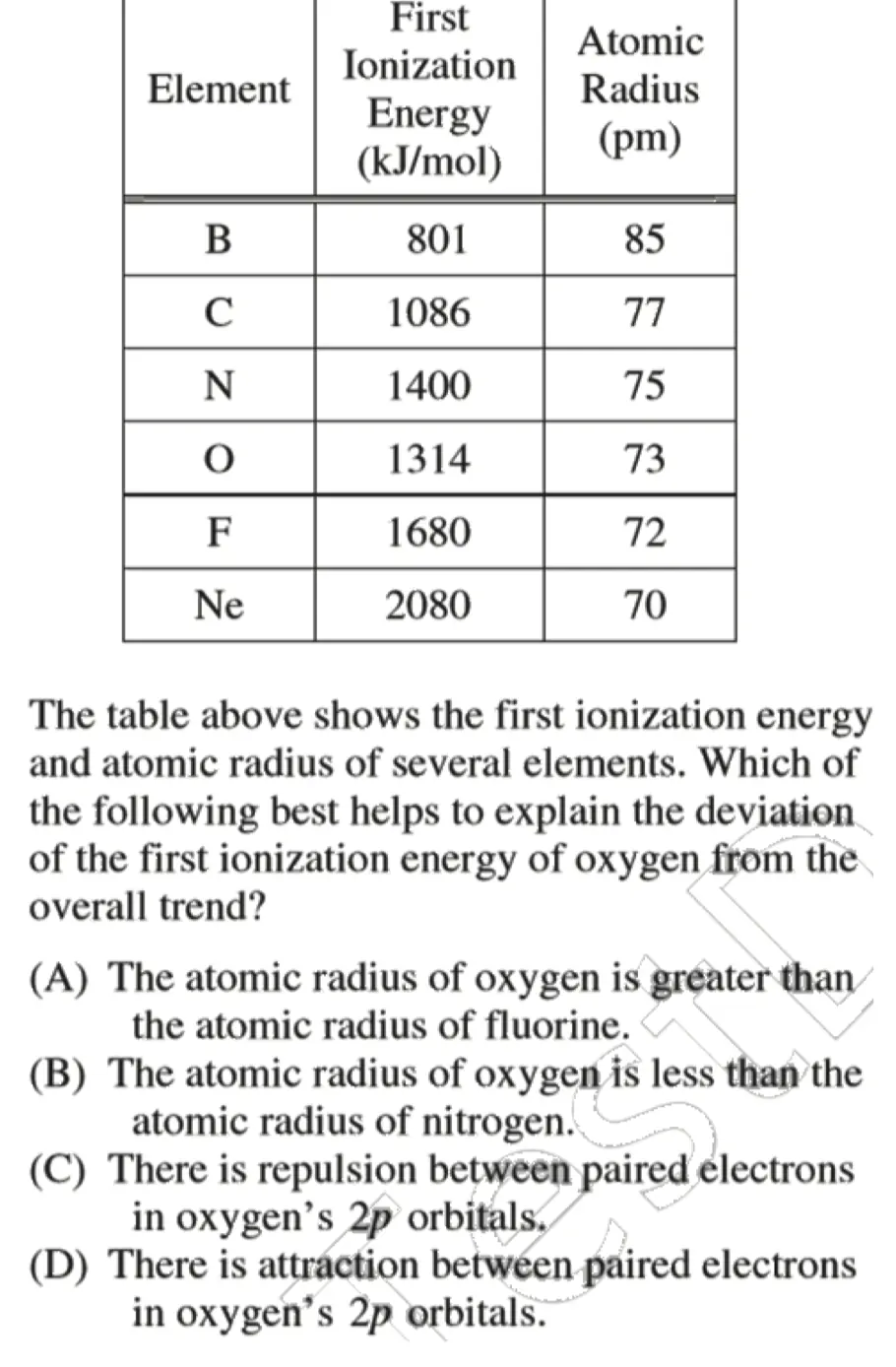
- AP 2014:
C
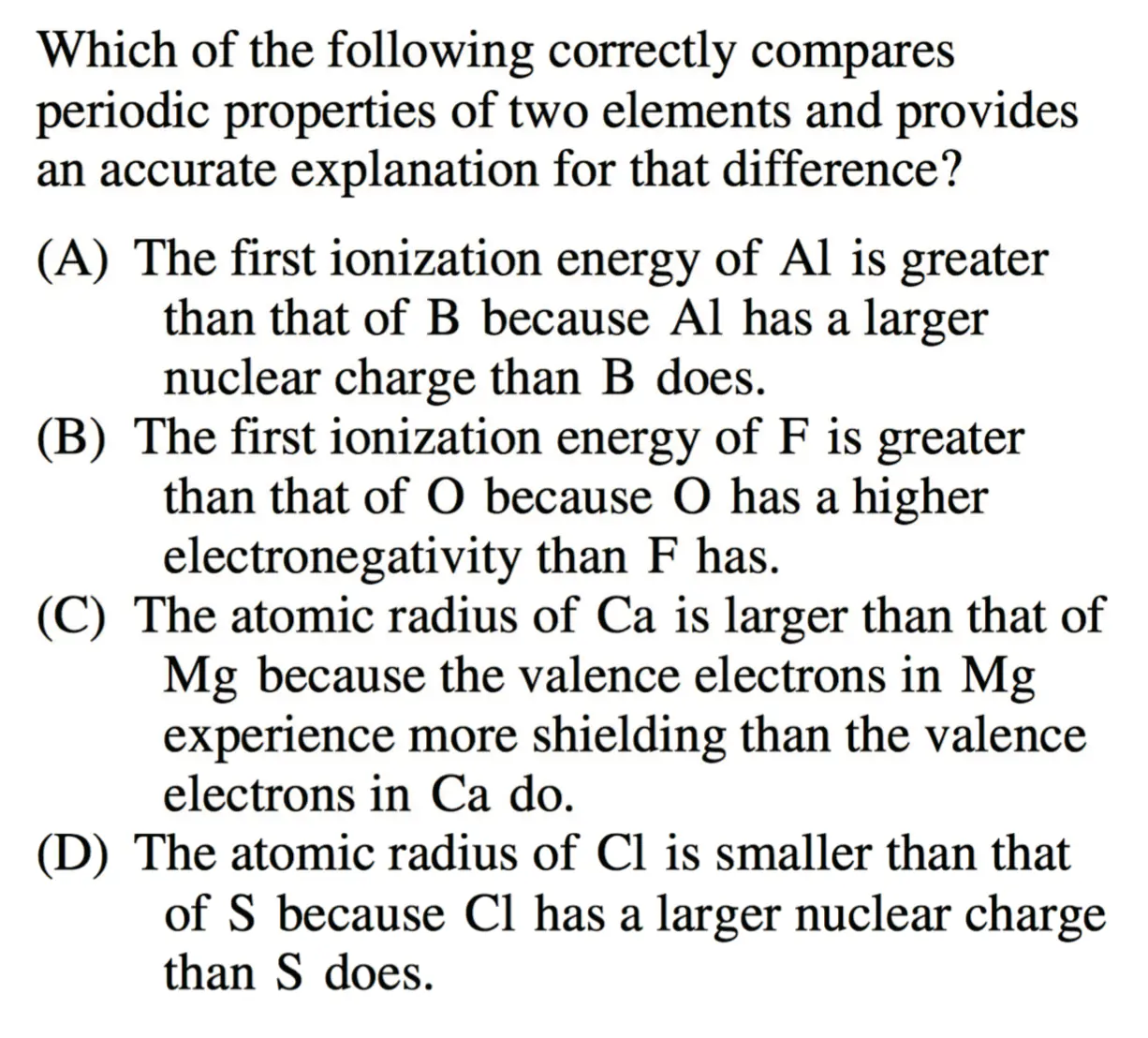
- Practice:
D
Electronegativity 电负性
电负性(Electronegativity)可以认为是原子得电子(成键)的能力
不考虑稀有气体
趋势与电离能同向
从左到右: 增加
- 质子数更多,对整体吸引力越大,所以得电子能力越强
由上到下: 下降
- 占用了更多电子壳层,半径变大,对电子吸引力越小
Practice:
C

Electron Affinity 电子亲和能
原子得到电子所对应的能量变化就是电子亲和能(Electron Affinity)
规律性不强
从左到右: 增加 (不完全规律)
- 质子数更多,对整体吸引力越大,所以得电子能力越强
由上到下: 下降 (只对第一主族)
- 占用了更多电子壳层,半径变大,对电子吸引力越小
